What is in this leaflet
This leaflet answers some common questions about ACARBOSE VIATRIS. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking this medicine against the benefits they expect it will have for you.
As ACARBOSE VIATRIS is a prescription medicine, it should only be used under medical supervision.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What ACARBOSE VIATRIS is used for
ACARBOSE VIATRIS tablets contain the active drug acarbose. They are used for the treatment of diabetes.
Acarbose helps to control your blood sugar levels in conjunction with diet, exercise, weight loss and other measures by slowing down the digestion of carbohydrates (complex sugars) from your diet. This reduces the abnormally high blood sugar levels that occur after each meal.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
Before you take ACARBOSE VIATRIS
When you must not take it
Do not take ACARBOSE VIATRIS if you have an allergy to:
- acarbose, the active ingredient in ACARBOSE VIATRIS
- any of the ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin.
Do not take ACARBOSE VIATRIS if:
- you have a severe kidney disorder (creatinine clearance <25 mL/min).
- you suffer from intestinal obstruction, inflammation or ulceration of the bowel, e.g. ulcerative colitis or Crohn’s disease.
- you have a hernia or have had previous abdominal surgery. If so, consult your doctor first.
Do not give this medicine to a child under the age of 18 years.
Do not take this medicine after the expiry date printed on the pack and blister. The expiry date is printed on the carton and on each blister after “EXP”. The expiry date refers to the last day of that month. If it has expired return it to your pharmacist for disposal.
Do not take this medicine if the packaging is torn or shows signs of tampering. If the packaging is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you are pregnant or intend to become pregnant. Like most medicines of this kind, acarbose is not recommended to be used during pregnancy. Your doctor will discuss the risks and benefits of using it if you are pregnant.
Tell your doctor if you are breast-feeding or planning to breast-feed. It is not known whether acarbose passes into breast milk.
If you have not told your doctor about any of the above, tell him/her before you start taking ACARBOSE VIATRIS.
Taking other medicines
Tell your doctor or pharmacist if you are taking other medicines, including any you have bought without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and ACARBOSE VIATRIS may interfere with each other. These include:
- Neomycin (Neosulf™)
- Colestyramine (Questran Lite™)
- Intestinal adsorbents (e.g. charcoal)
- Digestive enzyme preparations (Cotazym-S Forte™, Creon™, Pancrease™, Viokase™)
- Digoxin
(™ indicates trademark of the original manufacturer/supplier)
These medicines may be affected by ACARBOSE VIATRIS or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
You should avoid taking cane sugar (sucrose) and products containing sugar. They may cause stomach discomfort or even diarrhoea if taken while you are on ACARBOSE VIATRIS tablets.
If you are taking other medicines containing sulfonylureas or metformin or tend to have low blood sugar levels, tell your doctor before using ACARBOSE VIATRIS.
If you have not told your doctor or pharmacist about any of the above, tell them before you start taking ACARBOSE VIATRIS.
How to take ACARBOSE VIATRIS
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions printed on the pharmacist label, ask your doctor or pharmacist for help.
How much to take
To gain the maximum benefit from ACARBOSE VIATRIS it is important that you follow the prescribed diet as well as taking the exact dose prescribed by your doctor. This will help control your blood sugar levels and reduce side effects experienced.
At the start of treatment, your doctor may prescribe a low dose of ACARBOSE VIATRIS. Your doctor will determine the correct dose for you depending on your condition, other medicines and your response to the treatment. The average adult dose is one ACARBOSE VIATRIS 100 mg tablet taken three times daily.
How to take it
Swallow the tablets whole with a little liquid immediately before the meal. If you prefer not to swallow the tablets then chew the tablets with the first few mouthfuls of food.
When to take it
Take your medicine at about the same time each day with breakfast, lunch and dinner. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
When you first start your treatment, your doctor may recommend that you take your tablets once or twice a day, before increasing your dose to three times a day.
How long to take it
Do not stop taking the tablets unless you are told to do so by your doctor.
This medicine helps to control your condition, but does not cure it. It is important to keep taking your medicine even if you feel well.
If you do not feel well while you are taking them, see your doctor (see SIDE EFFECTS).
If you forget to take it
If you forget to take ACARBOSE VIATRIS at the time you are supposed to, do not take the tablets between meals. Wait until it is time for you to take your next dose and take ACARBOSE VIATRIS with your meal and continue as before. Do not take a double dose.
If you take too much (Overdose)
If you have taken too many ACARBOSE VIATRIS tablets, avoid foods or drinks containing carbohydrates and telephone your doctor or the Poisons Information Centre (Australia: 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much ACARBOSE VIATRIS. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
When ACARBOSE VIATRIS is taken with drinks and/or meals containing carbohydrates, overdosage can lead to diarrhoea and other intestinal symptoms such as flatulence (wind) and abdominal cramps.
While you are taking ACARBOSE VIATRIS
Things you must do
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking acarbose.
Tell any other doctors, dentists, and pharmacists who treat you that you are taking this medicine.
Take ACARBOSE VIATRIS exactly as instructed by your doctor. If you do not follow your doctor’s instructions, your blood sugar level may not be controlled.
Things you must not do
Do not take ACARBOSE VIATRIS to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop taking your medicine or lower the dosage without checking with your doctor. If you stop taking it suddenly, your condition may worsen or you may have unwanted side effects.
What to be careful of
Treating hypoglycaemia: (“hypos” or low blood sugar).
As a diabetic you may also be receiving other medicines for your diabetes. If ACARBOSE VIATRIS is prescribed for you in addition to sulfonylureas, metformin or insulin to control your diabetes, your doctor may need to adjust the dosages of these drugs to avoid the occurrence of “hypos” (low blood sugar levels).
When taking ACARBOSE VIATRIS together with these drugs, do not treat a “hypo” with ordinary sugar (sucrose). It will not work fast enough. Instead, you should take some GLUCOSE (also known as dextrose) tablets, honey, syrup or sweets which should be available from your local chemist.
SIDE EFFECTS
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking ACARBOSE VIATRIS.
All medicines have side effects. Sometimes they are serious; most of the time they are not.
In serious cases, you may need to obtain medical treatment.
Some patients may experience unwanted side effects during acarbose treatment.
These side effects are more common at the start of the treatment, but some may persist or develop after treatment, or when dosage of acarbose is adjusted by your doctor.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- flatulence (wind)
- abdominal “rumbling” noises
- a feeling of fullness
- softer stools or have diarrhoea; particularly if you have eaten foods containing sugar
The above list includes the more common side effects of your medicine. Normally these symptoms will disappear if you continue treatment and keep to your prescribed diet. These symptoms may get worse if you do not keep to your prescribed diet.
If your symptoms persist for more than 2 or 3 days, or if they are severe, consult your doctor or nearest emergency department particularly in the case of diarrhoea.
Do not take antacids to treat these symptoms as they are unlikely to give you any relief.
This list includes the less common side effects of your medicine.
- stomach cramps
- nausea
- indigestion
- vomiting
- increased appetite
- dizziness
- loss of appetite
Tell your doctor as soon as possible if you notice any of the following. This list includes rare side effects of your medicine.
- jaundice (yellowing of the skin)
- swelling from fluid retention
- inflammation of the liver (hepatitis)
- intestinal obstruction
- decrease in platelet count
- skin reactions such as redness, rash and urticaria (hives)
If while undergoing treatment with acarbose, you experience any side-effects or symptoms which you think may be due to this medication (whether or not it is mentioned in this leaflet) please inform your doctor or pharmacist as early as possible. You may need medical treatment in some cases.
ACARBOSE VIATRIS tablets may cause an increase in the results of certain liver function tests. Therefore, your doctor may conduct blood tests to monitor your liver function during the first 6 to 12 months of treatment with ACARBOSE VIATRIS.
These effects go away when treatment is stopped.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Other side effects not listed above may also occur in some people.
After taking ACARBOSE VIATRIS
When treatment with ACARBOSE VIATRIS is to be stopped, your prescribing doctor may need to alter the dose of other medication(s) accordingly and monitor your condition.
Storage
Keep your tablets in the pack until it is time to take them. If you take the tablets out of the pack they may not keep well.
Keep your tablets in a cool dry place where the temperature stays below 25°C. Protect from moisture.
Do not store it or any other medicine in the bathroom, near a sink, or on a window-sill.
Do not leave it in the car. Heat and damp can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over. Return any unused medicine to your pharmacist.
PRODUCT DESCRIPTION
What it looks like
ACARBOSE VIATRIS tablets are available in a blister pack of 90s in two strengths.
ACARBOSE VIATRIS 50 mg tablets are round, biconvex, white to off-white, marked with “ACA 50” on one side.
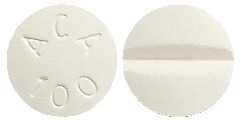
ACARBOSE VIATRIS 100 mg tablets are round, biconvex, white to off-white, marked with a score line on one side, and “ACA 100” on the reverse side.
Ingredients
Active ingredient per tablet:
ACARBOSE VIATRIS 50 mg – contains acarbose 50 mg
ACARBOSE VIATRIS 100 mg – contains acarbose 100 mg
Inactive ingredients:
- microcrystalline cellulose
- pregelatinised maize starch
- colloidal anhydrous silica
- magnesium stearate.
Supplier
ACARBOSE VIATRIS is supplied in Australia by:
Alphapharm Pty Limited trading as Viatris
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.viatris.com.au
Australian Registration Numbers
ACARBOSE VIATRIS 50 mg - AUST R 226796
ACARBOSE VIATRIS 100 mg - AUST R 226797
This document was prepared in
November 2022
Published by MIMS February 2023


 Soft stools are often produced by acarbose, but if the dosage of the individual case is too high, or after simultaneous ingestion of cane sugar, the stools can become unformed or even liquid. Should diarrhoea persist, patients should be closely monitored and the dosage reduced, or therapy withdrawn, if necessary.
Soft stools are often produced by acarbose, but if the dosage of the individual case is too high, or after simultaneous ingestion of cane sugar, the stools can become unformed or even liquid. Should diarrhoea persist, patients should be closely monitored and the dosage reduced, or therapy withdrawn, if necessary.

 Acarbose Viatris contains acarbose which is a complex oligosaccharide of microbial origin. Acarbose is made up of an unsaturated cyclitol unit, an amino sugar and a maltose residue. Acarbose is a white or yellowish powder with a molecular weight of 645.6. Acarbose is very soluble in water and has a pKa of 5.1. The empirical formula is C25H43NO18.
Acarbose Viatris contains acarbose which is a complex oligosaccharide of microbial origin. Acarbose is made up of an unsaturated cyclitol unit, an amino sugar and a maltose residue. Acarbose is a white or yellowish powder with a molecular weight of 645.6. Acarbose is very soluble in water and has a pKa of 5.1. The empirical formula is C25H43NO18.