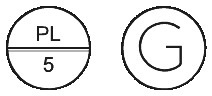What is in this leaflet
This leaflet answers some common questions about Barbloc.
It does not contain all of the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have benefits and risks. Your doctor has weighed the risks of you taking Barbloc against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, talk to your doctor or pharmacist.
Keep this leaflet with your medicine. You may need to read it again.
What Barbloc is used for
Barbloc is used to:
- lower high blood pressure, also called hypertension
- prevent angina
- treat irregular heart beat (arrhythmia).
Hypertension
Everyone has blood pressure. This pressure helps get your blood all around your body. Your blood pressure may be different at different times of the day, depending on how busy or worried you are. You have hypertension (high blood pressure) when your blood pressure stays higher than is needed, even when you are calm and relaxed.
There are usually no symptoms of hypertension. The only way of knowing that you have hypertension is to have your blood pressure checked on a regular basis. You may feel fine and have no symptoms, but eventually hypertension can cause stroke, heart disease and kidney failure.
Angina
Angina is a pain or uncomfortable feeling in the chest, often spreading to the arms or neck and sometimes to the shoulders and back. This may be caused by too little blood and oxygen getting to the heart. The pain of angina is usually brought on by exercise or stress but it can also happen while you are resting. Barbloc helps to prevent angina from happening. It is not used to treat an attack of angina once it starts.
Irregular heart beat
Irregular heartbeat, also known as arrhythmia, means that there is a disturbance of the normal rhythm or beat of the heart. Arrhythmias may be caused by a number of factors, including some heart diseases, an overactive thyroid gland, or chemical imbalances. Barbloc helps to restore the normal rhythm of the heart.
Barbloc belongs to a group of medicines called beta-blockers. It works by affecting the body's response to certain nerve impulses, especially in the heart. As a result, it decreases the heart's need for blood and oxygen and reduces the amount of work that the heart has to do. It also widens the blood vessels in the rest of the body, causing blood pressure to fall. Barbloc also helps the heart to beat more regularly.
Barbloc can be used alone or in combination with other medicines to treat your condition.
Your doctor may have prescribed Barbloc for another reason. Ask your doctor if you have any questions about why Barbloc has been prescribed for you.
Barbloc is not recommended for use in children, as there is not enough information on its effects in children.
Barbloc is available only with a doctor's prescription.
There is no evidence that Barbloc is addictive.
Before you take Barbloc
When you must not take it
Do not take Barbloc if you have an allergy to:
- any medicines containing pindolol
- any other beta-blocker medicine
- any of the ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin.
Do not take Barbloc if you have any of the following health problems/medical conditions:
- a history of bronchial asthma, wheezing or difficulty breathing, chronic cough or other severe lung problems
- a history of allergic problems, including hay fever
- irregular or a very slow heartbeat, less than 45 to 50 beats per minute
- an alteration in the structure and function of the right ventricle of the heart caused by a primary disorder of respiratory system (called "Cor pulmonale")
- sudden loss of consciousness in the past
- chest pain, mainly occurring when at rest
- severe heart disease or certain other heart conditions
- severe blood flow disturbances of your blood vessels causing paleness or poor circulation in the arms and legs
too much acid in your blood (metabolic acidosis).Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to honey bee or wasp stings, or to any other medicines, foods, preservatives or dyes. Your doctor will want to know if you are prone to allergies. Beta-blocker medicines can make an allergic reaction worse.
Tell your doctor if you have or have had any of the following health problems/medical conditions:
- diabetes mellitus (sugar diabetes)
- an overactive thyroid gland
- severe kidney problems
- certain types of angina such as Prinzmetal angina (also known as variant angina)
- milder forms of circulatory disturbances of blood vessels (conditions marked e.g. by paleness, cold hands or feet)
- phaeochromocytoma (a rare tumour of the adrenal gland) which is not being treated already with other medicines
- psoriasis (a skin disease characterised by thickened patches of red skin, often with silvery scales)
- shock or severely low blood pressure.
Tell your doctor if you are pregnant or plan to become pregnant. Barbloc may affect your baby, especially if you take it in the last stages of pregnancy. Your doctor will discuss the risks and benefits of taking Barbloc during pregnancy.
Tell your doctor if you are breastfeeding or wish to breastfeed. Very small amount of Barbloc passes into breast milk and could affect your baby.
If you have not told your doctor about any of the above, tell them before you start taking Barbloc.
Taking other medicines
Tell your doctor if you are taking any other medicines, including any that you get without a prescription from a pharmacy, supermarket or health food shop.
Some medicines may be affected by Barbloc, or may affect how well it works. These include:
- other beta-blocker medicines including eye drops
- other medicines used to treat high blood pressure such as calcium channel blockers or calcium antagonists
- clonidine, a medicine used to treat high blood pressure
- disopyramide, quinidine, and other medicines used to treat irregular heart beat (arrhythmias)
- insulin, and oral medicines to treat diabetes
- medicines used to treat high blood pressure, chest pain (angina pectoris), disturbances of heart rhythm
- digoxin, a medicine used to treat heart failure
- digitalis glycosides
- medicines commonly used during surgery or in emergency situations, such as dopamine, noradrenaline (norepinephrine), and certain anaesthetics
- medicines containing adrenaline (noradrenalin) or similar substances that raise blood pressure, such as those found in some nose and eye drops, cough medicines, or remedies for the common cold
- non-steroidal anti-inflammatory drugs (known as NSAIDs), which are medicines used to relieve pain or inflammation or to treat arthritis
- ergot alkaloids, a class of medicines used in the prevention and treatment of migraine headaches
- monoamine oxidase inhibitors, a class of medicines used to treat depression
- cimetidine, a medicine used to relieve heartburn and
- gastrointestinal ulcers
Your doctor can tell you what to do if you are taking any of these medicines.
If you are not sure whether you are taking any of these medicines, check with your doctor or pharmacist. Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking Barbloc.
How to take Barbloc
Follow all directions given to you by your doctor and pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the bottle, ask your doctor or pharmacist for help.
How much to take
Your doctor will tell you how many tablets you need to take each day and when to take them. This depends on your condition, on how you respond to this medicine and whether or not you are taking any other medicines.
The dose of this medicine will be different for different patients. Also, the number of doses you take each day and the time allowed between doses depend on the condition for which you are taking Barbloc.
Treatment is usually started with the lowest dose. Depending on how you respond to the treatment, your doctor may suggest a higher or lower dose.
For high blood pressure, the usual dose is 15 mg per day but can range from 10 mg to 30 mg per day. Doses above 15 mg should be divided into two or three smaller doses.
For angina, the usual dose is from 7.5 mg to 20 mg each day, divided into three doses.
For irregular heartbeat (arrhythmia), the usual dose is from 10 mg to 30 mg each day, divided into three doses.
Patients with kidney problems may need smaller doses.
Follow all directions given to you by your doctor and pharmacist carefully.
How to take it
Swallow the tablets with a glass of water.
Barbloc tablets can be broken in half if your doctor has prescribed half a tablet.
When to take it
Take Barbloc at about the same time each day.
How long to take it for
Do not stop taking this medicine without first checking with your doctor. Your doctor may want to gradually reduce the amount of medicine you are taking before stopping it completely. This helps to reduce the chance of your condition becoming worse and to prevent other unwanted effects on your heart.
Continue taking Barbloc as your doctor tells you.
Your doctor will check your progress to make sure the medicine is working and will discuss with you how long your treatment should continue.
If you forget to take it
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take the missed dose as soon as you remember, and then go back to taking your tablets as you would normally.
Do not take a double dose to make up for the dose you missed. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much Barbloc (overdose)
Immediately telephone your doctor, or the Poisons Information Centre (telephone 13 11 26), or go to Accident and Emergency at the nearest hospital, if you think you or anyone else may have taken too much Barbloc. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
If you take too much Barbloc, you may feel dizzy, sick (nausea), vomit, have a slow heart beat and even collapse.
While you are taking Barbloc
Things you must do
Before starting any new medicine, tell your doctor or pharmacist that you are taking Barbloc.
Tell all the doctors, dentists and pharmacists who are treating you that you are taking Barbloc.
If you become pregnant while taking Barbloc, tell your doctor.
If you have an allergic reaction to a food, another medicine or an insect sting while you are taking Barbloc, tell your doctor immediately.
If you have a history of allergies, there is a chance that Barbloc may make allergic reactions worse or harder to treat.
If you are being treated for diabetes, make sure you check your blood sugar regularly and report any problems to your doctor. Barbloc may change how well your diabetes is controlled. It may also prevent some of the warning signs of low blood sugar, such as fast heart beat, and may make low blood sugar last longer. The dose of your diabetes medicines may need to be changed.
If you plan to have surgery, including dental surgery, and will need an anaesthetic, tell your doctor or dentist that you are taking Barbloc. This will help your doctor to prevent unwanted side effects such as a sudden drop in blood pressure.
If this medicine makes you feel light-headed, dizzy or faint, be careful when getting up from a sitting or lying position. These symptoms may be due to a sudden fall in your blood pressure.
If this problem does not go away, talk to your doctor.
To avoid symptoms of low blood pressure, here are some hints that may help:
- stand up slowly to help your body get used to the change in position and blood pressure
- if you feel dizzy, sit or lie down until you feel better
- if you feel faint, breathe deeply and bend forward with your head between your knees
- take extra care when exercising, driving or standing for long periods, especially in hot weather. Drink plenty of fluids, especially if you sweat a lot.
Visit your doctor regularly so they can check on your progress.
Things you must not do
Do not stop taking Barbloc, or change the dose, without checking with your doctor. Stopping Barbloc suddenly may worsen your angina or irregular heartbeat, or cause other heart problems. Your doctor may want you to gradually reduce the amount of Barbloc you are taking before stopping completely.
Do not let yourself run out of Barbloc over the weekend or on holidays.
Do not use this medicine to treat any other conditions unless your doctor tells you to.
Do not give this medicine to anyone else, even if they have the same condition as you.
Things to be careful of
Be careful driving or operating machinery until you know how Barbloc affects you. Barbloc may cause drowsiness, dizziness or lightheadedness in some people. If any of these occur, do not drive, operate machinery or do anything else that could be dangerous.
Be careful getting up from a sitting or lying position. Dizziness, lightheadedness or fainting may occur, especially when you get up quickly. Getting up slowly may help.
Make sure you drink enough water in hot weather and during exercise when you are taking Barbloc, especially if you sweat a lot. If you do not drink enough water while taking Barbloc, you may feel faint or lightheaded or sick. This is because your blood pressure is dropping suddenly. If you continue to feel unwell, tell your doctor.
Be careful to dress warmly during cold weather, especially if you will be outside for a long time. Like other beta-blocker medicines, Barbloc may make you more sensitive to cold temperatures, especially if you have problems with your blood circulation. These medicines tend to decrease blood circulation in the skin, fingers and toes.
Talk to your doctor if you experience eye problems (dry, gritty, or burning eyes), Barbloc can be used by older people at the same dose as for younger people. Older people may experience more side effects than young people, and so might be monitored closely by their doctor.
Lifestyle measures that help reduce heart disease risk
By following these simple measures, you can further reduce the risk from heart disease.
- Quit smoking and avoid second-hand smoke.
- Limit alcohol intake.
- Enjoy healthy eating by:
- eating plenty of vegetables and fruit;
- reducing your saturated fat intake (eat less fatty meats, full fat dairy products, butter, coconut and palm oils, most take-away foods, commercially-baked products). - Be active. Progress, over time, to at least 30 minutes of moderate-intensity physical activity on 5 or more days each week. Can be accumulated in shorter bouts of 10 minutes duration. If you have been prescribed anti-angina medicine, carry it with you when being physically active.
- Maintain a healthy weight.
- Discuss your lifestyle and lifestyle plans with your doctor.
- For more information and tools to improve your heart health, call Heartline, the Heart Foundation's national telephone information service, on 1300 36 27 87 (local call cost).
Know warning signs of heart attack and what to do:
- Tightness, fullness, pressure, squeezing, heaviness or pain in your chest, neck, jaw, throat, shoulders, arms or back.
- You may also have difficulty breathing, or have a cold sweat or feel dizzy or light headed or feel like vomiting (or actually vomit).
- If you have heart attack warning signs that are severe, get worse or last for 10 minutes even if they are mild, call triple zero (000). Every minute counts.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Barbloc.
Like all other medicines, Barbloc may have unwanted side effects in some people. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
If you are over 65 years of age, you may have an increased chance of getting side effects.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if you notice any of the following and they worry you:
- tiredness, drowsiness, decreased alertness
- dizziness or light-headedness (sometimes with fainting), especially on standing up
- shakiness or trembling
- headache or other aches and pains
- disturbed sleep, vivid dreams
- feeling depressed
- stomach upset (mainly nausea or feeling sick) or vomiting
- diarrhoea or abdominal discomfort
- runny, itchy, red, dry or irritated eyes
- excess sweating
- sleep disturbances
- muscle cramps
Tell your doctor as soon as possible if you notice any of the following:
- skin reactions (rash, itching, hives, flaking of skin, worsening of psoriasis)
- abnormal thinking, or hallucinations (seeing or hearing things that are not there)
- numbness
If any of the following happen, tell your doctor immediately or go to Accident and Emergency at your nearest hospital:
- serious signs of allergy such as swelling of the face, lips or tongue which may cause problems with swallowing or breathing
- coldness, burning, tingling or numbness in arms and legs
- changes in heart rate, such as abnormally slow heart beat (called bradycardia), irregular heart beat or palpitations
- disturbed heart rhythm (called cardiac conduction disorder)
- weakness, hunger, trembling, flushing or paleness
- convulsions, fits or seizures
- chest tightness, wheezing, rattly breathing
- sudden, oppressive chest pain
- shortness of breath, sometimes with tiredness, weakness and reduced ability to exercise, swelling of the feet or legs due to fluid build up
- difficulty breathing with coughing or wheezing.
Tell your doctor if you notice anything that is making you feel unwell.
Other side effects not listed above may also occur in some patients.
After taking Barbloc
Storage
Keep your tablets in the bottle until it is time to take them. If you take the tablets out of the bottle they may not keep well.
Keep your tablets in a cool dry place where the temperature stays below 30°C.
Do not store Barbloc or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
Barbloc comes in 2 strengths of tablets:
- Barbloc 5 - round, white scored tablet marked PL over 5 on one side and G on the reverse. Each bottle of Barbloc 5 contains 100 tablets
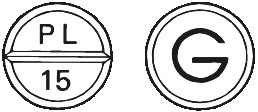
- Barbloc 15 - round, white scored tablet marked PL over 15 on one side and G on the reverse. Each bottle of Barbloc 15 contains 50 tablets.
Ingredients
The active ingredient in Barbloc is pindolol. Each Barbloc tablet contains 5 mg or 15 mg of pindolol.
The tablets also contain:
- microcrystalline cellulose
- pregelatinised maize starch
- colloidal anhydrous silica
- magnesium stearate
The tablets are gluten free.
Manufacturer
Barbloc is made in Australia by:
Alphapharm Pty Limited
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.mylan.com.au
Australian registration numbers:
Barbloc 5 - Aust R 17589
Barbloc 15 - Aust R 17588
This leaflet was prepared in August 2019.
Published by MIMS October 2019