What is in this leaflet
This leaflet answers some common questions about Cardizem CD capsules and Cardizem tablets. It does not contain all the available information.
It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking these medicines against the benefits they expect it will have for you.
Keep this leaflet with the medicine. You may need to read it again.
What these medicines are used for
You have been prescribed either Cardizem CD capsules or Cardizem tablets. These medicines both contain an active ingredient called diltiazem hydrochloride. The difference between these is that Cardizem CD is designed to release the active ingredient slowly so that it works over 24 hours and can be taken once a day (CD stands for "controlled delivery"). Cardizem tablets release the active ingredient faster and so must be taken more often (3-4 times a day, as your doctor has instructed).
These medicines belong to a group of medicines called calcium channel blockers or calcium antagonists. They work by opening up blood vessels, which lowers blood pressure and lets more blood and oxygen reach the heart. They do not change the amount of calcium in your blood or bones.
Cardizem tablets are used to prevent angina.
Cardizem CD capsules are used to prevent angina or to treat hypertension (high blood pressure).
Angina is a pain or uncomfortable sensation in the chest, often spreading to the arms or neck and sometimes to the shoulders and back. The pain of angina is due to a shortage of oxygen to the heart.
High blood pressure can have many different causes, including kidney disease, hardening of the arteries and some hormone imbalances. However, the vast majority of people with high blood pressure have no identifiable cause for it. If left untreated, high blood pressure can lead to serious health problems such as a stroke or heart attack.
Your doctor may have prescribed these medicines for another reason.
Ask your doctor if you have any questions about why these medicines have been prescribed for you.
There is no evidence that these medicines are addictive.
These medicines are available only with a doctor's prescription.
Before you take these medicines
When you must not take them:
Do not take these medicines:
If you have had any of the following medical conditions:
- certain types of abnormal heart rhythm
- hypotension (low blood pressure)
- heart attack or other heart-related complications
- pulmonary congestion (fluid on the lungs)
If you are currently taking any of the following medications:
- dantrolene (muscle relaxant)
- ivabradine (an antiviral)
If you have an allergy to:
- diltiazem hydrochloride or any of the ingredients listed at the end of this leaflet
Symptoms of an allergic reaction to these medicines may include:
- asthma, wheezing or shortness of breath
- swelling of the face, lips or tongue, which may cause difficulty in swallowing or breathing
- hives, itching or skin rash
- fainting
If you are pregnant, or intend to become pregnant. These medicines may affect your developing baby if they are taken during pregnancy.
If you are breastfeeding or intend to breastfeed. The active ingredient of these medicines passes into breast milk and may affect your baby.
If the packaging is torn or shows signs of tampering or if tablets or capsules do not look quite right.
If the expiry date (EXP) printed on the pack has passed. If you take this medicine after the expiry date has passed, it may not work as well.
If you are not sure whether you should start taking these medicines, contact your doctor.
Do not give these medicines to a child. The safety and effectiveness of these medicines have not been established in children.
Before you start to take it
Tell your doctor:
If you have any allergies to:
- any other medicines
- any other substances, such as foods, preservatives or dyes
If you are pregnant or intend to become pregnant. Cardizem CD capsules and Cardizem tablets should not be used during pregnancy.
If you are breastfeeding or plan to breastfeed. Your doctor will discuss this situation with you. A decision will have to be made whether to discontinue breastfeeding or discontinue therapy taking into consideration the importance of the medicine.
If you have or have had any medical conditions, especially the following:
- abnormal heart beat rhythm
- hypotension (low blood pressure)
- heart attack or other heart-related complications
- impaired renal (kidney) or hepatic (liver) function
- diabetes
- asthma
Taking other medicines
Tell your doctor if you are taking any other medicines, including any that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interfere with Cardizem CD capsules or Cardizem tablets. These include:
- dantrolene (a muscle relaxant)
- aspirin
- medications used to help prevent blood clots (antiplatelets)
- cilostazol (a medicine used to treat blockage of blood vessels to the legs)
- Some other medicines for your heart or high blood pressure (eg. beta blockers, digoxin, amiodarone, nitrates)
- ciclosporin, which you may have been given after an operation or because of rheumatoid arthritis
- rifampicin (an antibiotic)
- cimetidine or ranitidine (for ulcers or reflux)
- diazepam (for depression, alcohol withdrawal or anxiety)
- phenytoin (for epilepsy)
- carbamazepine (for bipolar disorder or epilepsy)
- lithium (for bipolar disorder)
- theophylline (for asthma and other breathing problems) certain drugs used to treat prostate problems
- ivabradine (an antiviral)
- inhaled anaesthetic agents such as halothane, isoflurane, enflurane (for surgery)
- drugs used to lower your blood cholesterol (including simvastatin, lovastatin)
- benzodiazepines or medicines used as sedatives or to treat anxiety such as midazolam, triazolam
- corticosteroids such as methylprednisolone, prednisone, cortisone
- antiarrhythmics or medicines used to treat irregular heart beats
- medicines used during scans to see images of your body
Cardizem CD capsules or Cardizem tablets may themselves be affected, or they may affect how well these medicines work. You may need to take different amounts of your medicine, or you may need to take different medicines. Your doctor will advise you.
Your doctor and pharmacist may have more information on medicines to be careful with or to avoid while taking Cardizem CD capsules or Cardizem tablets.
How to take these medicines
How much to take
Cardizem CD capsules can be taken once a day, preferably at the same time every day. Cardizem tablets can be taken three or four times a day. Your doctor will tell you how often and how much Cardizem CD or Cardizem tablets to take. Follow all directions given to you by your doctor and pharmacist carefully. Write them down if necessary.
If you do not understand the instructions on the packaging of these medicines, ask your doctor or pharmacist for help.
How to take it
Swallow the capsules or the tablets with a glass of water. Do not chew them.
When to take it
Take these medicines at the same time(s) every day.
How long to take it
If you are not sure how long to take your medicine, talk to your doctor.
If you forget to take it
If you are taking these medicines for angina, do not suddenly stop taking them since this can cause severe angina for a day or two.
If you forget to take a dose and it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to. Otherwise, take it as soon as you remember, and then go back to taking your tablets or capsules as you would normally.
Do not take a double dose to make up for the dose that you missed.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
If you think that you or anyone else may have taken too much of these medicines, immediately telephone your doctor or Poisons Information Centre (Australia telephone 13 11 26, New Zealand telephone 0800 764 766), or go to the Accident and Emergency Department at your nearest hospital. Do this even if there is no signs of discomfort or poisoning.
If you take too much of these medicines, you may:
- feel continuously light-headed or dizzy
- notice your heart beating very slowly
- feel pain, which could be severe, in your left arm and chest.
If any of these occur, you should get medical attention immediately.
While you are using these medicines
Things you must do
Take these medicines exactly as your doctor has prescribed.
If you do not follow your doctor's instructions, you may not get relief from your attacks of angina, or your blood pressure may not be as well controlled as it could be.
If you are taking these medicines for angina, tell your doctor if you continue to have angina attacks or if they become more frequent.
Tell all your doctors, dentists and pharmacists that you are taking these medicines.
Tell your doctor or pharmacist that you are taking Cardizem CD capsules or Cardizem tablets if you are about to be started on any new medicine.
Things you must not do
Do not use these medicines to treat any other complaints unless your doctor says to.
Do not give these medicines to anyone else, even if they have the same condition as you.
As mentioned previously, if you are taking these medicines for angina, do not suddenly stop taking your medicine since this can cause severe angina for a day or two.
Things to be careful of
Be careful driving or operating machinery until you know how these medicines affect you. These medicines may cause dizziness and fainting in some patients, especially when you first start to use them. Make sure you know how you react to these medicines before you drive a car, operate machinery, or do anything else that could be dangerous if this happens to you.
Be careful not to overdo physical activities when you first start using these medicines. You may feel better when you start taking these medicines, but you will need time to improve your physical fitness.
Drinking grapefruit juice may increase the effects of Cardizem and Cardizem CD.
Get up slowly when getting out of bed or standing up if you feel light-headed, dizzy or faint. If this is a problem and it gets worse or continues, talk to your doctor.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are using these medicines.
These medicines help most people with angina, and Cardizem CD will help control most people's blood pressure, but they may have unwanted effects in a few people.
All medicines can have side effects. Sometimes they are serious, but most of the time they are not. You may need medical treatment if you get some of the side effects.
Ask your doctor or pharmacist any questions you may have.
Tell your doctor if you notice any of the following and they worry you:
- swelling or flushing (feeling hot suddenly)
- headache
- nausea, vomiting, constipation, diarrhoea, indigestion, gastric pain
- dizziness
- confusion, hallucinations, abnormal dreams, mental depression or mood changes
- trouble sleeping
- nervousness, tremor
- ringing or other persistent noise in the ears
- loss of memory
- dry mouth
- loss of appetite
- weight increase
- increased sensitivity to the sun
- unusual movements or uncontrollable movements
- rash or an itchy, burning or prickly sensation
- small round, raised itchy areas on the skin
- weakness or tiredness
These side effects are usually mild.
Tell your doctor immediately if you notice any of the following:
- you feel continuously light headed or dizzy
- you notice your heart beating irregularly, slowly or very quickly
- you feel pain, which may be severe, in your left arm and chest
- you have blisters and bleeding in the lips, eyes, mouth, nose or genitals
- you have skin reactions such as red, painful or itchy spots, blisters or peeling of the skin.
- you have difficulty breathing, wheezing or coughing
These are serious side effects. You may need urgent medical attention. Serious side effects are rare.
Other side effects not listed above may also occur in some patients. Tell your doctor if you notice anything else that is making you feel unwell.
Ask your doctor or pharmacist if you do not understand anything in this section.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
After taking these medicines
Storage
Keep these medicines in their container until it is time to take them. If you take the medicine out of its container it may not keep well.
Keep these medicines in a cool, dry place where it stays below 25°C. Do not store them, or any other medicine, in a bathroom or near a sink. Do not leave them in the car or on a windowsill. Heat and dampness can destroy some medicines.
Keep this medicine where young children cannot reach it. A locked cupboard at least one-and-a half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking your Cardizem medication, or it has passed its expiry date, ask your pharmacist what to do with any tablets or capsules that are left over.
Product description
What your medicine looks like
Cardizem CD capsules
Cardizem CD capsules are available in packs of 30 for the 180 mg and 240 mg strengths. Each blister strip is calendarised and contains 15 capsules. For your first dose, take the capsule labelled "Take this Capsule First". Take the next doses according to the day of the week which is printed on the blister foil.
The 360 mg strength is available in a bottle of 30 capsules.
All doses (180 mg, 240 mg and 360 mg) are two component capsules:
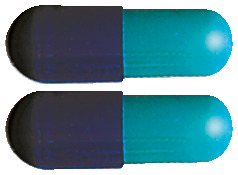
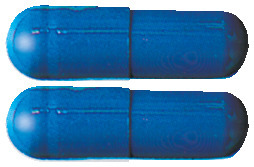
- The 180 mg capsules are blue and light turquoise
- The 240 mg capsules are blue
- The 360 mg capsules are white and light blue
Cardizem tablets
Cardizem tablets are supplied in plastic bottles of 90 tablets with a seal in the mouth of the bottle. The tablets are light yellow, speckled, round and biconvex. The word "Marion" is engraved on one side of the tablet while the other side is scored and engraved with "1772".
Ingredients
Cardizem CD 180 and 240 mg capsules contain:
Diltiazem hydrochloride 180 mg or 240 mg, fumaric acid, purified talc, non-pareil seeds (sucrose), colloidal anhydrous silica, white beeswax, ethylcellulose, castor oil, stearic acid, methacrylic acid copolymers, tributyl acetylcitrate, simethicone, gelatin, brilliant blue FCF, black iron oxide and titanium dioxide.
Cardizem CD 360 mg capsules contain:
Diltiazem hydrochloride 360 mg, non-pareil seed (sucrose), povidone, sodium lauryl sulfate, diethyl pthalate, purified talc, methacrylic acid copolymers, tributyl acetylcitrate, simethicone, titanium dioxide, brilliant blue FCF, titanium dioxide and gelatin.
Cardizem tablets contain:
Diltiazem hydrochloride 60 mg, lactose monohydrate, microcrystalline cellulose, hypromellose, colloidal anhydrous silica, magnesium stearate, methyl hydroxybenzoate, sunset yellow FCF CI 15985, quinoline yellow CI 47005 and a film-coating (Opadry YS-5-7044 & methylhydroxybenzoate).
Manufacturer/Distributor
Cardizem CD capsules and Cardizem tablets are distributed by:
sanofi-aventis australia pty ltd
12-24 Talavera Road
Macquarie Park
NSW 2113
Australia
sanofi-aventis new zealand limited
Level 8, 56 Cawley Street
Ellerslie, Auckland, New Zealand
Australian Registration Numbers:
The following products are available:
Cardizem CD capsules
180 mg: AUST R 46818
240 mg: AUST R 46822
360 mg: AUST R 75251*
Cardizem tablets
60 mg: AUST R 73179*
*denotes not available in New Zealand
This leaflet was prepared in September 2017
cardizem-cd-ccdsv11-cmiv15-sep17
Published by MIMS November 2017



 Chemically, diltiazem hydrochloride is the hydrochloride salt of (2S,3S)-5-(2-dimethylaminoethyl)- 2,3,4,5-tetrahydro- 2-(4-methoxyphenyl)-4-oxo- 1,5-benzothiazepin-3-yl acetate. It has a molecular weight of 450.98.
Chemically, diltiazem hydrochloride is the hydrochloride salt of (2S,3S)-5-(2-dimethylaminoethyl)- 2,3,4,5-tetrahydro- 2-(4-methoxyphenyl)-4-oxo- 1,5-benzothiazepin-3-yl acetate. It has a molecular weight of 450.98.