What is in this leaflet?
Please read this leaflet carefully before you take CLAVAM tablets.
This leaflet answers some common questions about CLAVAM tablets. It does not contain all of the available information.
It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Sometimes new risks are found even when a medicine has been used for many years. Your doctor has weighed the expected benefits of you taking CLAVAM tablets against the risks this medicine could have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine.
You may need to read it again.
What are CLAVAM tablets used for?
CLAVAM tablets contain two active ingredients. One of these is a penicillin called amoxycillin and the other is clavulanic acid. CLAVAM tablets belong to the penicillin group of antibiotics.
CLAVAM tablets are used for the short term treatment of wide range of infections caused by bacteria. These infections may affect the chest (e.g. bronchitis or pneumonia), bladder (e.g. cystitis), sinuses (e.g. sinusitis), the ears (e.g. otitis media) or the skin.
CLAVAM tablets work by killing the bacteria that cause these infections. CLAVAM tablets will not work against infections caused by viruses such as colds or the flu.
Your doctor may have prescribed it for another reason. If you want more information ask your doctor.
This medicine is available only with a doctor’s prescription.
There is no evidence that it is addictive.
Before you take CLAVAM Tablets
Do not take CLAVAM Tablets if:
- you have had an allergic reaction to penicillin or similar types of antibiotics (such as cephalosporins) or any of the ingredients contained in CLAVAM tablets. The ingredients are listed at the end of this leaflet. Some of the symptoms of an allergic reaction may include skin rash, itching, difficulty in breathing and swelling of the face or tongue.
- you have previously experienced liver problems after taking CLAVAM tablets or any other medicines.
- The expiry date (EXP) printed on the pack has passed.
- The packaging is torn or shows signs of tampering.
Do not give this medicine to anyone else; your doctor has prescribed it specifically for you and your condition.
If you are not sure whether you should start taking CLAVAM tablets, talk to your doctor.
Tell your doctor if:
- you have ever had an allergic reaction (such as a rash) to antibiotics or other substances in the past.
- you are allergic to foods, dyes, preservatives or any other medicines.
- you have experienced liver problems after taking CLAVAM tablets or any other medicines.
- you have glandular fever (mononucleosis) or leukaemia.
- you are pregnant or think you may be pregnant or are breast-feeding. Your doctor will discuss with you the possible risks and benefits of taking CLAVAM tablets during pregnancy or while you are breast- feeding. CLAVAM tablets can pass to your baby from breast milk.
- you have any kidney or liver problems. The dosage of CLAVAM tablets may need to be changed or you may need to be given an alternative medicine.
- you have to test your urine for sugar. CLAVAM tablets may affect the results of these tests.
- Tell your doctor or pharmacist if you are taking any other medicines, including medicines that you buy without a prescription from your pharmacy, supermarket or health food shop. In particular tell your doctor or pharmacist if you are taking any of the following:
- warfarin or other medicines used to prevent blood clots
- mycophenolate
- Medicines used to treat gout (eg. probenecid or allopurinol).
- other antibiotics. These may interfere with the actions of CLAVAM tablets.
- The contraceptive pill. As with other antibiotics, you may need to use extra birth control methods (eg. condoms) while taking CLAVAM tablets. - Some medicines may affect the way other medicines work. Your doctor or pharmacist will be able to tell you what to do when taking CLAVAM tablets with other medicines.
How to take CLAVAM Tablets
Follow the directions given to you by your doctor and pharmacist. Their directions may differ from the information contained in this leaflet.
Please read the direction label carefully. If you have any concerns about how to take this medicine talks to your doctor or pharmacist.
How much to take:
Take as directed by your doctor or Pharmacist.
The usual dose of CLAVAM is twice daily.
How to take it:
Swallow the CLAVAM tablet with a full glass of water or other liquid. CLAVAM tablets can also be broken in half, but should not be chewed.
CLAVAM tablets should be taken immediately before or with the first mouthful of food.
CLAVAM tablets work best when taken this way. It may also help to prevent stomach upsets. However, CLAVAM tablets will still work if they are taken without food.
Space the doses as evenly as possible throughout the day. If you are taking CLAVAM tablets twice a day, take a dose about every twelve hours.
How long to take it for:
Keep taking CLAVAM tablets until the course is finished or for as long as your doctor tells you.
Do not stop taking CLAVAM tablets just because you feel better as the infection can return.
Do not stop taking, or change the dose without first checking with your doctor.
If you forget to take it:
If your next dose is due within six hours, skip the dose you missed and take your next dose at the normal time. Otherwise, take the missed dose as soon as you remember and then go back to taking your tablets as directed by your doctor.
Do not take a double dose to make up for the dose that you have missed. Taking more than the prescribed dose can increase the chance of unwanted side-effects.
What do I do if I take too much? (Overdose)
Immediately telephone your doctor or Poisons Information Centre (telephone 131126) for advice, if you think you or anyone else may have taken too much, even if there are no signs of discomfort or poisoning.
Symptoms of overdose include mild to severe nausea, vomiting, cramps and diarrhoea.
If you are not sure what to do, contact your doctor, pharmacist or nearest hospital. Be sure to show the doctor the CLAVAM pack.
While you are taking CLAVAM tablets
Things you must do:
Take CLAVAM tablets exactly as your doctor has prescribed.
Tell your doctor if, for any reason you have not taken your medicine exactly as directed. Otherwise, your doctor may think that it was not working as it should and change your treatment unnecessarily.
Tell your doctor, dentist or pharmacist you are taking CLAVAM tablets before starting any other medicines. Some medicines may affect the way other medicines work.
Tell your doctor if the symptoms of your infection become worse, or do not improve within a few days of starting CLAVAM tablets.
Things you must not do:
Do not give this medicine to anyone else, even if their symptoms seem similar to yours.
Do not use to treat any other complaints unless your doctor says to.
Things to be careful of:
Alcohol should be avoided during and for several days after treatment with CLAVAM tablets. Some people who drink alcohol while taking antibiotics similar to CLAVAM tablets have experienced adverse effects.
Be careful driving or operating machinery until you know how CLAVAM tablets affect you.
Generally, these tablets do not cause any problems with your ability to drive a car or operate machinery.
However, as with many other medicines, CLAVAM tablets may cause dizziness or tiredness in some people.
If you develop severe diarrhea either when taking CLAVAM tablets or within several weeks after treatment, tell your doctor as soon as possible. Do not take any medication to stop the diarrhoea (eg. Lomotil or Imodium).
What are the side-effects?
Check with your doctor as soon as possible if you think you are experiencing any side-effects or allergic reactions due to taking CLAVAM tablets, even if the problem is not listed below.
Like other medicines, CLAVAM can cause some side-effects. If they occur, they are most likely to be minor and temporary. However, some may be serious and need medical attention.
Tell your doctor about any effect which is troublesome or ongoing.
MILD EFFECTS
- Tell your doctor if you notice any of the following that are troublesome or ongoing:
- diarrhoea (several loose bowel movements per day), indigestion, pain in the stomach, feeling sick or being sick
- white, furry, sore tongue and mouth (oral thrush), abnormal taste
- soreness or itching of the vagina or vaginal discharge (vaginal thrush)
- aseptic meningitis, headache, dizziness, tiredness, hot flushes
- tooth discolouration
- unusually active (hyperactivity)
MORE SERIOUS EFFECTS
- Tell your doctor immediately if you notice any of the following during or after taking CLAVAM tablets:
- itching, rash
- dark urine or pale stools
- yellowing of the skin or eyes (jaundice)
- severe stomach cramps
- severe watery or bloody diarrhoea
- unusual bleeding or bruising
These may be symptoms of rare serious side-effects and require urgent medical attention. - Stop taking CLAVAM tablets and immediately contact your doctor or go to the emergency department of your nearest hospital if any of the following happens:
Wheezing, hives, severe skin reaction, fainting, swelling of limbs, face, lips, mouth or throat, difficulty swallowing or breathing, joint discomfort or swelling, swollen lymph glands, nausea and vomiting or fever.
These may be signs of an allergic reaction or other reaction to CLAVAM tablets.
- Allergy to these antibiotics is rare.
- Fits/seizures. - Rare events that have been reported with CLAVAM tablets include:
- inflammation of the bowel (colitis)
- inflammation of the liver (hepatitis)
- inflammation of the kidney (nephritis)
- blood disorders
- crystals in the urine (crystalluria)
Remember, you should tell your doctor or pharmacist as soon as possible if any of these, or any other unusual events or problems occur during or after treatment with CLAVAM Tablets.
This is not a complete list of all possible side-effects. Others may occur in some people and there may be some side-effects not yet known.
Tell your doctor or pharmacist if you notice any side-effects from your medicine which are not mentioned here.
Do not be alarmed by this list of possible side-effects. You may not experience any of them.
How do I store CLAVAM tablets?
Keep your tablets in the original pack until it is time to take them. If you take the tablets out of the pack they may not keep well.
Keep the pack in a cool dry place. Do not leave it in the car on a hot day. Do not store medicine in the bathroom or near a sink. Heat and dampness can destroy CLAVAM tablets.
Store all medicines out of the reach of children, such as in a locked cupboard.
If your doctor tells you to stop taking CLAVAM tablets or if the expiry date has passed, ask your pharmacist what to do with any tablets that are left over.
Product description
What they look like:
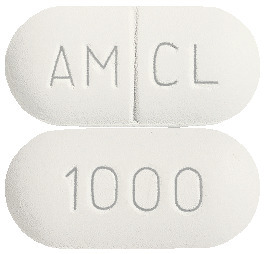
CLAVAM tablets come as White to off whit capsule shaped, biconvex, film coated tablets debossed with AM and CL separated by break line on one side and 1000 on other side.
Other ingredients:
CLAVAM tablets contain the following inactive ingredients: Microcrystalline Cellulose, Sodium Starch Glycolate, Colloidal anhydrous Silica, magnesium Stearate, Insta Moistshield [PI 108592], Isopropyl Alcohol and Dichloromethane.
CLAVAM tablets are available only with a doctor's prescription.
CLAVAM tablets come in Blister packs of 10 and HDPE pack of 20`s
Supplier
Your CLAVAM tablets are supplied by:
PHARMACOR PTY. LTD
SUITE 803, LEVEL 8, TOWER A, THE ZENITH, 821 PACIFIC HIGHWAY.CHATSWOOD, NSW, 2067,
Australia
Australian Registration Numbers for CLAVAM 875mg/125mg tablets is -
Blister Packs AUST R 202571
Bottle Packs AUST R 202577
Where to go for further Information
Pharmaceutical companies are not in a position to give people an individual diagnosis or medical advice. Your doctor or pharmacist is the best person to give you advice on the treatment of your condition.
The information provided applies only to: CLAVAM tablets.
Date of First Inclusion In Australian Register of Therapeutic Goods (The ARTG)
14/11/2013
This document was last updated in
Sept 2020
Published by MIMS March 2021






 The in vitro data in Table 5 are available but their clinical significance is unknown.
The in vitro data in Table 5 are available but their clinical significance is unknown.


 During clinical trials, the most frequently reported adverse events related or possibly related to amoxicillin and potassium clavulanate 500 mg/125 mg tablet therapy were diarrhoea (12.8%), nausea (5.2%), headache (4.8%), abdominal pain (4.5%).
During clinical trials, the most frequently reported adverse events related or possibly related to amoxicillin and potassium clavulanate 500 mg/125 mg tablet therapy were diarrhoea (12.8%), nausea (5.2%), headache (4.8%), abdominal pain (4.5%).