What is in this leaflet
This leaflet answers some common questions about Inza tablets.
It does not contain all the available information.
It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking Inza against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What Inza is used for
Inza belongs to a group of medicines called Non-Steroidal Anti-Inflammatory Drugs (NSAIDs).
Inza relieves pain and inflammation (swelling, redness and soreness) that may occur in the following:
- in different types of arthritis including rheumatoid arthritis, osteoarthritis and ankylosing spondylitis
- in muscle and bone injuries such as sprains, strains, lower back pain (lumbago), rheumatism and tendonitis, such as tennis elbow
- swelling and pain after setting broken or dislocated bones
- menstrual cramps (period pain)
- headache, including migraines
- following surgery
- dental pain
Although Inza can relieve the symptoms of pain and inflammation, it will not cure your condition.
Your doctor may have prescribed Inza for another reason.
Ask your doctor if you have any questions about why Inza has been prescribed for you.
This medicine is available only with a doctor's prescription.
Inza is not addictive.
Before you take Inza
When you must not take it
Do not take Inza if you have an allergy to:
- Inza or any of the ingredients listed at the end of this leaflet.
- aspirin or any other NSAID medicine
Many medicines used to treat headache, period pain and other aches and pains contain aspirin or NSAID medicines. If you are not sure if you are taking any of these medicines, ask your pharmacist.
Symptoms of an allergic reaction to these medicines may include:
- asthma, wheezing or shortness of breath
- swelling of the face, lips or tongue which may cause difficulty in swallowing or breathing
- hives, itching or skin rash
- fainting
If you are allergic to aspirin or NSAID medicines and take Inza, these symptoms may be severe.
Do not take Inza if:
- you are vomiting blood or material that looks like coffee grounds
- you are bleeding from the rectum (back passage), have black sticky bowel motions (stools) or bloody diarrhoea
- you currently have a peptic ulcer (i.e. stomach or duodenal ulcer), or have had one before
- you have severe liver disease
- you have recently had or are about to have heart bypass surgery
- you are taking other medications which contain naproxen or naproxen sodium (e.g. Naprogesic®, Anaprox® or Naprosyn®)
- you have severe heart failure
Do not give Inza to a child under the age of 2 years. The safety and effectiveness of Inza in children under 2 years of age group has not been established.
Do not take Inza if the package is torn or shows signs of tampering.
Do not take Inza is the expiry date (EXP) printed on the pack has passed. If you take this medicine after the expiry date has passed, it may not work as well.
If you are not sure if you should start taking Inza, talk to your doctor.
Before you start to take it
Tell your doctor if:
- you have any allergies to:
- any other medicines including aspirin or other NSAID medicines
- any other substances, such as foods, preservatives or dyes. - you are pregnant or intend to become pregnant
Inza may impair fertility and is not recommended in women attempting to conceive. Inza may affect your developing baby if you take it during pregnancy.
If it is necessary for you to take Inza, your doctor will discuss the risks and benefits of taking it during pregnancy. - you are breastfeeding or intend to breast-feed.
Inza passes into breast milk. The effect on the baby is not known. - you have or have had any medical conditions, especially the following:
- heartburn, indigestion, stomach ulcers or other stomach problems
- vomiting blood or bleeding from the back passage
- bowel or intestinal problems such as ulcerative colitis
- kidney or liver problems
- heart failure
- high blood pressure or heart problems
- swelling of the ankles or feet
- a tendency to bleed or other blood problems, such as anaemia - you currently have an infection
If you take Inza while you have an infection, the signs may be hidden (e.g. pain, fever). This may make you think, mistakenly, that you are better or that it is not serious. - you plan to have surgery
Inza can prolong bleeding
If you have not told your doctor about any of the above, tell them before you take any Inza.
Taking other medicines
Tell your doctor if you are taking any other medicines including any that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interfere with Inza. These include:
- antacids, medicines used to treat indigestion and heartburn
- aspirin, salicylates or other NSAID medicines
- colestyramine, a medicine used to treat high cholesterol levels
- diuretics, also called fluid or water tablets,
- lithium, a medicine used to treat some types of depression
- probenecid, a medicine used to treat gout
- phenytoin, a medicine used to treat epilepsy
- methotrexate, a medicine used to treat arthritis and some cancers
- sucralfate, a medicine used to treat and prevent stomach ulcers
- warfarin, a medicine used to prevent blood clots
- heparin, a medicine used to prevent blood clots
- medicines used to treat high blood pressure including ACE inhibitors, angiotensin receptor antagonists and betablockers
- some medicines used to treat diabetes
- sodium bicarbonate, a medicine used to treat stomach upset or ulcers
- steroids, medicines used to treat inflammation
- serotonin reuptake inhibitors, also known as (SSRIs), medicines used to treat some types of depression
- zidovudine, a medicine used to treat HIV infection.
These medicines may be affected by Inza, or may affect how well it works. You may need to use different amounts of your medicine, or you may need to take different medicines. Your doctor will advise you.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking Inza.
Ask your doctor or pharmacist if you are not sure about this list of medicines.
Use in Children
There is no specific information available to recommend the use of Inza in children under 5 years.
Use in People Over 65 Years
Older people may be at more risk of developing stomach ulcers and hence your doctor may prescribe a lower dose.
How to take Inza
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
How much to take
Take Inza exactly as your doctor has prescribed. Your doctor will tell you how many Inza tablets you need to take each day. If you are an older patient, your doctor may give you a lower dose
Sprains, strains and period pain
The recommended dose is 500 mg given initially, then 250 mg every 6 to 8 hours as needed. The total dose in one day should not exceed 1250 mg.
Migraine Headache
The recommended dose is 750 mg taken at the first sign of a migraine. An additional dose of 250 mg to 500 mg can be taken at least an hour after the initial dose, if required. The total dose in one day should not exceed 1250 mg.
Arthritis
The recommended dose is 375 mg to 1000 mg a day, divided in two doses.
How to take it
Swallow the tablets whole with a full glass of water or milk.
When to take it
Take the tablets during or immediately after food with a full glass of water or milk. This may help reduce the possibility of an upset stomach.
How long to take Inza
Do not take Inza for longer than your doctor says.
Depending on your condition, you may need to use Inza only once, for a few days, a few weeks or for longer periods.
For sprains and strains, Inza is usually only needed for a few days.
If you are taking Inza for arthritis, it will not cure your condition but it should help to control pain, swelling and stiffness. If you have arthritis, Inza should be taken every day for as long as your doctor prescribes.
Ask your doctor if you are not sure how long to take Inza for.
If you forget to take Inza
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to. Otherwise, take it as soon as you remember and continue taking it as you would normally.
Do not double a dose to make up for the dose you have missed.
If you have trouble remembering your dose, ask your pharmacist for some hints.
If you are not sure what to do, ask your doctor or pharmacist.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at your nearest hospital if you think that you or anyone else may have taken too much Inza. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
If you take too much Inza, you may experience drowsiness, pain or tenderness in the stomach, stomach upset including nausea (feeling sick), vomiting, heartburn, indigestion or cramps.
While you are taking Inza
Things you must do
If you become pregnant while taking Inza, tell your doctor immediately.
Tell all the doctors, dentists and pharmacists who are treating you that you are taking Inza.
Ask your doctor and pharmacist before you start taking any new medicines.
If you are going to have surgery, tell your doctor that you are taking Inza.
If you are going to have any laboratory tests, tell your doctor that you are taking Inza. Inza may affect the results of some of these tests.
If you get an infection while using Inza, tell your doctor. Inza may hide some of the signs of an infection and may make you think, mistakenly that you are better or that it is not serious. Signs of an infection may include fever, pain, swelling and redness.
Tell your doctor if, for any reason, you have not taken your medicine exactly as prescribed. Otherwise, your doctor may think that it was not effective and change your treatment unnecessarily.
Tell your doctor if you feel the tablets are not helping your condition.
Things you must not do
Do not give Inza to anyone else, even if they have the same condition as you.
Do not use Inza to treat any other complaints unless your doctor tells you to.
Things to be careful of
Be careful driving or operating machinery until you know how Inza affects you. As with other NSAID medicines, Inza may cause drowsiness or light-headedness in some people. Make sure you know how you react to Inza before you drive a car, operate machinery, or do anything else that could be dangerous if you are dizzy or light-headed. If this occurs do not drive. If you drink alcohol, dizziness or light-headedness may be worse.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Inza.
Inza helps most people with pain due to inflammation, but it may have unwanted side effects in a few people.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- stomach upset, including nausea (feeling sick), indigestion, heartburn
- loss of appetite
- constipation, diarrhoea, pain in the stomach
- dizziness, light-headedness
- headache, drowsiness,
- buzzing or ringing in the ears
- sore or dry mouth or tongue
- feeling thirsty
- aching muscles, muscle tenderness or weakness, not caused by exercise
These side effects of Inza are usually mild.
Tell your doctor immediately if you notice any of the following:
- bleeding or bruising more easily than normal, reddish-purplish or blue-black blotches under the skin
- eye problems such as blurred vision, sore eyes, itching
- severe or persistent headache
- fast or irregular heartbeats, also called palpitations
- difficulty hearing, deafness
- unusual weight gain, swelling of the ankles or legs
- severe skin rashes
- yellowing of the eyes or skin (jaundice)
These are serious side effects. You may need medical attention. Serious side effects are rare.
Tell your doctor immediately, or go to Accident and Emergency at your nearest hospital if you experience any of the following:
- vomiting blood or what looks like coffee grounds
- bleeding from your back passage (rectum), black sticky bowel motions (stools) or bloody diarrhoea
- difficulty breathing, wheezing or shortness of breath
- swelling of the face, lips or tongue which may cause difficulty in swallowing or breathing
- severe dizziness, spinning sensation
- severe pain or tenderness in any part of the stomach
- sudden or severe itching, skin rash and hives
- pain, tightness in the chest
These are very serious side effects. You may need urgent medical attention or hospitalisation. These side effects are very rare.
This is not a complete list of all possible side effects. Others may occur in some people and there may be some side effects not yet known.
Tell your doctor if you notice anything else that is making you feel unwell, even if it is not on this list.
Ask your doctor or pharmacist if you don't understand anything in this list.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
After taking Inza
Storage
Keep your tablets in the bottle until it is time to take them. If you take the tablets out of the bottle, they will not keep well.
Keep the tablets in a cool dry place where the temperature stays below 30°C.
Do not store Inza, or any other medicine in a bathroom or near a sink.
Do not leave it in the car or on window sills. Heat and dampness can destroy some medicines.
Keep Inza where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Do not keep your tablets in the refrigerator.
Disposal
If your doctor tells you to stop taking Inza, or the tablets have passed their expiry date, ask your pharmacist what to do with any that are left over.
Product description
What it looks like
Inza comes in 2 strengths of tablets:
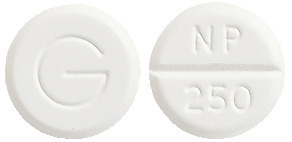
- Inza 250 - round, white, scored tablet marked NP/250 and G
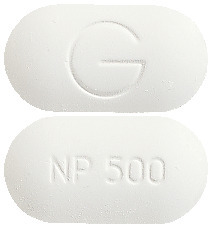
- Inza 500 - oblong, white tablet marked NP 500 and G.
Each bottle contains 50 tablets.
Ingredients
The active ingredient in Inza is naproxen:
- each Inza 250 tablet contains 250 mg of naproxen
- each Inza 500 tablet contains 500 mg of naproxen.
The tablets also contain the following inactive ingredients:
- lactose monohydrate
- microcrystalline cellulose
- maize starch
- purified talc
- colloidal anhydrous silica
- pregelatinized maize starch
- sodium starch glycollate
- magnesium stearate.
The tablets contain excipients of known effects including galactose, sugars and sulfites. The tablets are gluten free.
Manufacturer
Inza is made in Australia by:
Alphapharm Pty Limited
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point
NSW 2000
www.mylan.com.au
Australian registration numbers:
Inza 250 - Aust R 40927
Inza 500 - Aust R 40929
This leaflet was prepared on 24 July 2019.
Inza_cmi\July19 /00
Published by MIMS September 2019

 Chemical name: (+)-6-methoxy-alpha-methyl-2-naphthaleneacetic acid.
Chemical name: (+)-6-methoxy-alpha-methyl-2-naphthaleneacetic acid.