SUMMARY CMI
NEXIUM®
Consumer Medicine Information (CMI) summary
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
1. Why am I using NEXIUM?
NEXIUM contains the active ingredient esomeprazole magnesium trihydrate. NEXIUM is used to treat Reflux Oesophagitis, Upper gastrointestinal symptoms associated with non-steroidal anti-inflammatory drugs (NSAIDs) therapy, Peptic Ulcers Associated with Helicobacter pylori Infection, Zollinger-Ellison Syndrome and Bleeding Peptic Ulcers.
For more information, see Section 1. Why am I using NEXIUM? in the full CMI.
2. What should I know before I use NEXIUM?
Do not use if you have ever had an allergic reaction to NEXIUM or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding.
For more information, see Section 2. What should I know before I use NEXIUM? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with NEXIUM and affect how it works.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
4. How do I use NEXIUM?
- NEXIUM is available as either tablets or as granules for suspension in sachets. The dose of NEXIUM tablets is usually 20 mg or 40 mg a day. The dose of NEXIUM sachets is 10 mg to 20 mg a day. The dose for tablets or sachets depends on what condition you are being treated for and how severe it is.
- Follow all directions given to you by your doctor and pharmacist carefully.
More instructions can be found in Section 4. How do I use NEXIUM? in the full CMI.
5. What should I know while using NEXIUM?
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using NEXIUM? in the full CMI.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. These include nausea or vomiting, constipation, diarrhoea, headache, wind, stomach pain, skin rash, itchy skin, dizziness or dry mouth. However, some serious side effects may need urgent medical attention. These include shortness of breath, wheezing or difficulty breathing, swelling of the face, lips, tongue or other parts of the body, severe skin reaction which may include rash, itching, redness, blistering or peeling of the skin, signs of liver inflammation including yellowing of the skin or eyes, feeling generally unwell, nausea, vomiting, loss of appetite, blood in the urine and increased or decreased urine output.
For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
FULL CMI
NEXIUM®
Active ingredient: esomeprazole magnesium trihydrate
Consumer Medicine Information (CMI)
This leaflet provides important information about using NEXIUM. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using NEXIUM.
Where to find information in this leaflet:
1. Why am I using NEXIUM?
2. What should I know before I use NEXIUM?
3. What if I am taking other medicines?
4. How do I use NEXIUM?
5. What should I know while using NEXIUM?
6. Are there any side effects?
7. Product details
1. Why am I using NEXIUM?
NEXIUM contains the active ingredient esomeprazole magnesium trihydrate. NEXIUM is a type of medicine called a proton-pump inhibitor. It works by decreasing the amount of acid made by the stomach, to give relief of symptoms and allow healing to take place. This does not stop food being digested in the normal way.
Reflux Oesophagitis
NEXIUM is used to treat reflux oesophagitis. This can be caused by "washing back" (reflux) of food and acid from the stomach into the food pipe (oesophagus). Reflux can cause a burning sensation in the chest rising up to the throat, also known as heartburn. NEXIUM is also taken to help stop reflux oesophagitis coming back or relapsing.
Upper gastrointestinal symptoms associated with non-steroidal anti-inflammatory drugs (NSAIDs) therapy
NEXIUM is taken to treat the symptoms of pain or discomfort, in the stomach caused by NSAIDs, a type of medicine for pain or inflammation.
NEXIUM is also taken to help heal and prevent ulcers caused by NSAIDs.
Peptic Ulcers Associated with Helicobacter pylori Infection
Most people who have a peptic (gastric and duodenal) ulcer also have a bacterium called Helicobacter pylori in their stomach.
Depending on the position of the ulcer it is called a gastric or duodenal ulcer. A gastric ulcer occurs in the stomach. A duodenal ulcer occurs in the duodenum which is the tube leading out from the stomach. If you have a peptic ulcer, your doctor will prescribe NEXIUM with antibiotics. When NEXIUM and antibiotics are taken together, they work to kill the bacterium and let your ulcer heal. You may need further treatment with antibiotics.
Zollinger-Ellison Syndrome
NEXIUM is also used to treat a rare condition called Zollinger-Ellison syndrome, where the stomach produces large amounts of acid, much more than in ulcers or reflux disease.
Bleeding Peptic Ulcers
When peptic ulcers become severe enough, they start to bleed. You may receive treatment injected into your veins initially. This treatment may be followed with NEXIUM tablets or granules prescribed by your doctor for a longer period of time. This is to help your ulcer/s to heal.
2. What should I know before I use NEXIUM?
Warnings
Do not use NEXIUM if:
- you are allergic to esomeprazole magnesium trihydrate, or any of the ingredients listed at the end of this leaflet.
- Always check the ingredients to make sure you can use this medicine.
- you are allergic to any medicines containing proton-pump inhibitors.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin
Do not take NEXIUM if you are also taking atazanavir or cilostazol. Please check with your doctor or pharmacist if you are taking these medicines. These medicines will be affected by NEXIUM.
Check with your doctor if you have:
- allergies to any other medicines, foods, dyes or preservatives
- any problems with your liver
- severe kidney problems
- any other medical conditions
- been diagnosed with osteoporosis
- ever had a skin reaction after treatment with a medicine similar to NEXIUM that reduces stomach acid
There is no evidence that NEXIUM is addictive.
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant.
Talk to your doctor if you are breastfeeding or intend to breastfeed.
It is not known if it is safe for you to take NEXIUM while you are pregnant. It may affect your baby.
It is not known if your baby can take in NEXIUM from breast milk if you are breastfeeding.
Do not take NEXIUM if you are pregnant or breastfeeding unless your doctor says so. Ask your doctor about the risks and benefits involved.
Children younger than 1 year:
NEXIUM is not approved for use in children younger than 1 year of age. There is no specific information about use in children younger than 1 year of age, so NEXIUM is not recommended in these patients.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking or have recently been taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Do not take NEXIUM if you are taking the following medicines:
- atazanavir and nelfinavir - medicines used to treat viral infections such as HIV
- cilostazol - a medicine used to treat intermittent claudication
Some medicines may interfere with NEXIUM and affect how it works. These include:
- ketoconazole, itraconazole and voriconazole - medicines used to treat fungal infections
- diazepam - a medicine used to treat anxiety and some other conditions
- phenytoin - a medicine used to treat epilepsy or fits
- citalopram, clomipramine and imipramine - medicines used to treat depression
- St John's wort - a herbal remedy used to treat mood disorders
- clarithromycin and rifampicin - medicines used to treat bacterial infections
- warfarin and clopidogrel - medicines used to prevent blood clots
- digoxin - a medicine used to treat heart conditions
- methotrexate - a medicine used to treat arthritis and some types of cancer
- tacrolimus and mycophenolate mofetil - medicines used to assist in organ transplants
- erlotinib or related medicines used to treat cancer
These medicines may be affected by NEXIUM or may affect how well it works. You may need different amounts of your medicine, or you may need to take different medicines.
Your doctor can tell you what to do if you are taking any other medicines.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect NEXIUM.
4. How do I use NEXIUM?
How much to take
NEXIUM is available as tablets and as granules for suspension in sachets.
NEXIUM Tablets
- Take one NEXIUM tablet each day, unless your doctor has told you otherwise.
- The dose of NEXIUM tablets is usually 20 mg or 40 mg a day depending on what condition you are being treated for and how severe it is.
NEXIUM Sachets
NEXIUM sachets are usually used for children or adults who have trouble swallowing tablets. The dose of NEXIUM sachets is 10 to 20 mg a day depending on what condition is being treated and how severe it is.
When to take NEXIUM
- NEXIUM should be used at about the same time each day.
- Keeping a regular time for taking NEXIUM will help to remind you to take it.
- NEXIUM can be taken with food or on an empty stomach.
- Keep taking NEXIUM for as long as your doctor recommends.
- In most patients, NEXIUM relieves symptoms rapidly and healing is usually complete within 4 weeks.
How to take NEXIUM
Tablets: Swallow NEXIUM tablets whole with a glass of water. Do not crush or chew the tablets. If the tablets are chewed or crushed they will not work properly.
If you have difficulty swallowing the tablets:
- Place the tablet in half a glass of non-carbonated water. Mineral water or other liquids are not suitable.
- Gently mix the tablet and water by stirring, taking care not to crush the tablet.
- Stir until the tablet dissolves into little pellets.
- Drink the liquid with the pellets immediately, or within 30 minutes. Do not chew the pellets.
- Rinse the glass with half a glass of water and drink.
If you cannot swallow at all, follow steps 1-3 above to disperse the tablets and administer the liquid and pellets through a gastric tube.
Granules for oral suspension: Do not crush or chew the pellets in the suspension. If the pellets are chewed or crushed, they will not work properly.
The granules in NEXIUM sachets should be dispersed as follows in non-carbonated water (mineral water is not suitable) before being taken:
- For a 10 mg dose empty the contents of a 10 mg sachet into a glass containing 15 mL of water. For a 20 mg dose empty the contents of two 10 mg sachets into a glass containing 30 mL of water.
- Stir the contents and leave for a few minutes to thicken.
- Stir again and drink within 30 minutes.
- If any material remains after drinking, add more water, stir and drink immediately.
For patients who cannot swallow, NEXIUM granules for oral suspension can be administered via a large syringe through a nasogastric or gastric tube:
- For a 10 mg dose add the contents of a 10 mg sachet to a syringe containing 15 mL of water. For a 20 mg dose add the contents of two 10 mg sachets to a syringe containing 30 mL of water.
- Immediately shake the syringe and leave for a few minutes to thicken.
- Shake the syringe and inject through the nasogastric or gastric tube within 30 minutes.
- Refill the syringe with 15 mL of water, then shake and flush any remaining contents from the nasogastric or gastric tube into the stomach.
If you forget to use NEXIUM
NEXIUM should be used regularly at the same time each day. If you miss your dose at the usual time, take it as soon as you remember, and then go back to taking it as you would normally.
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Do not take a double dose to make up for the dose you missed.
If you use too much NEXIUM
If you think that you have used too much NEXIUM, you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre
(by calling 13 11 26), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while using NEXIUM?
Things you should do
Take NEXIUM exactly as your doctor has prescribed.
If you are about to start on any new medicine, tell your doctor, dentist or pharmacist that you are taking NEXIUM.
Tell all doctors, dentists and pharmacists who are treating you that you are taking NEXIUM.
Tell your doctor if you become pregnant while you are taking NEXIUM.
Tell your doctor if your symptoms return.
Although NEXIUM can heal ulcers successfully, it may not prevent them recurring at a later date.
If you need to have any medical tests while you are taking NEXIUM, tell your doctor.
It may affect the results of some tests.
Things you should not do
- Do not take it to treat any other complaints unless your doctor tells you to.
- Do not give this medicine to anyone else, even if their symptoms seem similar to yours.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how NEXIUM affects you.
Looking after your medicine
- Keep your NEXIUM in the blister pack or sachet until it is time to take it. If you take NEXIUM out of the blister pack or sachet it will not keep well.
- Keep it in a cool, dry place where the temperature stays below 25°C.
Follow the instructions in the carton on how to take care of your medicine properly.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on window sills.
Keep NEXIUM where young children cannot reach it.
A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
| Speak to your doctor if you have any of these less serious side effects and they worry you. |
| Speak to your doctor immediately if you have any of these side effects. These side effects may require medical attention. |
Serious side effects
| Serious side effects | What to do |
| Call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
| Contact your doctor immediately if you notice any of these side effects |
Occasionally, NEXIUM may be associated with changes in your liver or blood, which may require your doctor to do certain blood tests.
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What NEXIUM contains
| Active ingredient (main ingredient) | esomeprazole magnesium trihydrate |
| Other ingredients (inactive ingredients) | Tablets
Granules for oral suspension
|
| Potential allergens |
|
NEXIUM tablets and granules for oral suspension do not contain gluten.
Do not take this medicine if you are allergic to any of these ingredients.
What NEXIUM looks like
Tablets
NEXIUM 20 mg are light pink, oblong shaped tablets engraved 20 mg on one side and A / EH on the other. (AUST R 74133)
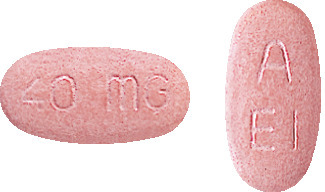
NEXIUM 40 mg are pink, oblong shaped tablets engraved 40 mg on one side and A / EI on the other. (AUST R 74134)
NEXIUM Granules for oral suspension
NEXIUM 10 mg granules for oral suspension are pale yellow fine granules (brownish granules may be visible) in a unit dose sachet. (AUST R 135726)
NEXIUM tablets are available in blister packs of 30 tablets.
NEXIUM granules for oral suspension are available in packs containing 30-unit dose sachets.
Who distributes NEXIUM
AstraZeneca Pty Ltd
ABN 54 009 682 311
66 Talavera Road
MACQUARIE PARK NSW 2113
Telephone:- 1800 805 342
This leaflet was prepared in April 2025.
NEXIUM® is a registered trade mark of the AstraZeneca group of companies.
©AstraZeneca 2025
VV-RIM-01378372 v15.0
Published by MIMS August 2025

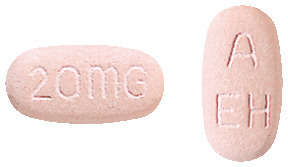

 Repeated dose administration of 5 mg esomeprazole resulted in insufficient exposure in 1 to 5 year olds.
Repeated dose administration of 5 mg esomeprazole resulted in insufficient exposure in 1 to 5 year olds.
 In vivo results demonstrate that acid control with esomeprazole is dose dependent and that it is significantly greater, more sustained and less variable compared to an equal dose of the racemate.
In vivo results demonstrate that acid control with esomeprazole is dose dependent and that it is significantly greater, more sustained and less variable compared to an equal dose of the racemate. In a five way crossover study, the 24 hour intragastric pH profile of oral esomeprazole 40 mg, lansoprazole 30 mg, omeprazole 20 mg, pantoprazole 40 mg and rabeprazole 20 mg once daily was evaluated in 34 symptomatic GORD patients. The results are tabulated in Table 6.
In a five way crossover study, the 24 hour intragastric pH profile of oral esomeprazole 40 mg, lansoprazole 30 mg, omeprazole 20 mg, pantoprazole 40 mg and rabeprazole 20 mg once daily was evaluated in 34 symptomatic GORD patients. The results are tabulated in Table 6. A 6 way crossover study was conducted to investigate the dose response relationship assessed by intragastric pH monitoring after repeated once daily oral doses of 20, 40 and 80 mg of esomeprazole and 20, 40 and 80 mg of pantoprazole in symptomatic GORD patients. Results are provided in Table 7.
A 6 way crossover study was conducted to investigate the dose response relationship assessed by intragastric pH monitoring after repeated once daily oral doses of 20, 40 and 80 mg of esomeprazole and 20, 40 and 80 mg of pantoprazole in symptomatic GORD patients. Results are provided in Table 7.
 Based on pooled data from all clinical trials in patients with baseline endoscopy grades B to D, healing rates at 4 and 8 weeks were statistically significantly better for esomeprazole 40 mg compared with omeprazole 20 mg.
Based on pooled data from all clinical trials in patients with baseline endoscopy grades B to D, healing rates at 4 and 8 weeks were statistically significantly better for esomeprazole 40 mg compared with omeprazole 20 mg.

 Based on the Quality of Life in Reflux and Dyspepsia questionnaire, patients on Nexium 20 mg gained significantly improved well-being (emotional distress dimension), sleep quality (sleep problem dimension) and improved ability to eat and drink (food/ drink problems dimension) compared to placebo. The GSRS questionnaire indicated significantly less reflux symptoms in both studies and significantly less abdominal pain and indigestion in one of the two studies.
Based on the Quality of Life in Reflux and Dyspepsia questionnaire, patients on Nexium 20 mg gained significantly improved well-being (emotional distress dimension), sleep quality (sleep problem dimension) and improved ability to eat and drink (food/ drink problems dimension) compared to placebo. The GSRS questionnaire indicated significantly less reflux symptoms in both studies and significantly less abdominal pain and indigestion in one of the two studies. Nexium IV treatment followed by the oral treatment regimen reduced the total number of days patients were hospitalised due to rebleeding during the 30 day treatment by 43% compared to placebo. Hospitalisations exceeding 5 days were observed in 4.8% of patients treated with Nexium compared to 10.5% for placebo.
Nexium IV treatment followed by the oral treatment regimen reduced the total number of days patients were hospitalised due to rebleeding during the 30 day treatment by 43% compared to placebo. Hospitalisations exceeding 5 days were observed in 4.8% of patients treated with Nexium compared to 10.5% for placebo.
