What is in this leaflet
This leaflet answers some common questions about NOTEN. It does not contain all of the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking NOTEN against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, talk to your doctor or pharmacist.
Keep this leaflet with your medicine. You may need to read it again.
What NOTEN is used for
NOTEN belongs to a group of medicines called beta-blockers.
It works by affecting the body's response to certain nerve impulses, especially in the heart. As a result, it decreases the heart's need for blood and oxygen and therefore reduces the amount of work the heart has to do. It widens the blood vessels in the body, causing blood pressure to fall. It also helps the heart to beat more regularly.
NOTEN is used to:
- lower high blood pressure, which is called hypertension
- prevent angina
- treat an irregular heart beat or rhythm, called arrhythmias
- treat heart attacks, or reduce the risk of heart complications following a heart attack
NOTEN may be either used alone or in combination with other medicines to treat your condition.
Hypertension
Everyone has blood pressure. This pressure helps to push blood all around your body. Your blood pressure changes during the day, depending on how busy you are or how you are feeling.
You have hypertension when your blood pressure stays higher than is needed, even when you are calm and relaxed.
Regular blood pressure checks are the only way of knowing that you have hypertension. There are usually no symptoms of hypertension and you may feel fine. If high blood pressure is not treated, serious health problems such as stroke, heart disease and kidney failure may occur.
NOTEN helps to lower your blood pressure.
Angina
Angina is a pain or uncomfortable feeling in the chest, often spreading to the arms or neck and sometimes to the shoulders and back. This may be caused by not enough blood and oxygen reaching your heart. The pain of angina is often brought on by exercise or stress, but it can also occur at rest.
NOTEN is used to prevent angina. It is not used to relieve a sudden attack of angina.
Irregular heart beat (arrhythmia)
An irregular heartbeat, also known as an arrhythmia, means that there is a disturbance of the heart's normal rhythm or beat. Arrhythmias may be caused by many factors, including some heart diseases, an overactive thyroid gland or chemical imbalances.
NOTEN helps restore your heart's normal rhythm.
Reducing heart complications after heart attack
After a heart attack, you may have complications such as an irregular heart beat or an increased chance of having another heart attack.
NOTEN helps to prevent these complications from occurring.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
NOTEN is not recommended for use in children, as there have been no studies of its effects in children.
There is no evidence that NOTEN is addictive.
This medicine is available only with a doctor's prescription.
Before you take NOTEN
When you must not take it
Do not take NOTEN if you have an allergy to:
- any medicine containing atenolol
- any of the ingredients listed at the end of this leaflet
- any other beta-blocker medicines
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin
Do not take NOTEN if you have:
- asthma, wheezing, difficulty breathing, bronchitis or other lung problems; or have had them in the past
- a history of allergic problems, including hay fever. Symptoms of an allergy may include rash, itching, watery eyes or sneezing.
- a very slow heartbeat, less than 45 to 50 beats per minute
- a severe blood vessel disorder causing poor circulation in the arms and legs
- certain other heart conditions, such as heart failure
- phaeochromocytoma (a rare tumour of the adrenal gland) that is not being treated with other medicines
- low blood pressure, also called hypotension
- too much acid in your blood (metabolic acidosis)
- you are receiving certain anaesthetics for medical or dental procedures
- you are receiving emergency treatment for shock or very low blood pressure
Do not take this medicine if you are pregnant or intend to become pregnant.
Do not take this medicine if you are breast feeding or intend to breast feed. Your doctor will discuss the possible risks and benefits of taking NOTEN during pregnancy and whilst breast feeding.
The active ingredient in NOTEN passes into breast milk and there is a possibility that your baby may be affected.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines (including eye drops), foods, preservatives, dyes or insect stings. NOTEN may make allergies worse or make them harder to treat.
Tell your doctor if you have or have had any of the following medical conditions:
- heart problems
- diabetes
- an overactive thyroid gland, called hyperthyroidism
- kidney problems
- any blood vessel disorders causing poor blood circulation in the arms and legs
If you have not told your doctor about any of the above, tell them before you start taking NOTEN.
Taking other medicines
Tell your doctor if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and NOTEN may interfere with each other. These include:
- other beta-blocker medicines
- medicines used to treat high blood pressure, angina or an irregular heart beat, such as verapamil or clonidine
- medicines used to treat other heart problems
- insulin and other medicines used to treat diabetes
- medicines used to treat arthritis, pain or inflammation such as indometacin or ibuprofen
- medicines commonly used during surgery or in emergency situations such as adrenaline (epinephrine), noradrenaline (norepinephrine) and certain anaesthetics
These medicines may be affected by NOTEN or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
How to take NOTEN
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the label, ask your doctor or pharmacist for help.
How much to take
Hypertension
The usual dose is from 50 mg (1 tablet) up to 200 mg (4 tablets) daily.
If your dose is 100 mg or less, you may take it once a day.
If you need to take more than 100 mg (2 tablets), take half of your dose in the morning and the other half at night.
Angina or arrhythmia (irregular heart beat)
The usual dose is from 50 mg daily (1 tablet) as a single dose up to 100 mg (2 tablets) either as a single dose or a divided dose given as 1 tablet in the morning and 1 tablet at night.
Heart attack
The usual dose is 50 mg (1 tablet) daily.
Certain people such as the elderly or those with kidney problems may require a lower dose.
How to take it
Swallow the tablets whole with a glass of water.
When to take it
Take your medicine at about the same time each day. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
It does not matter if you take NOTEN before or after food.
How long to take it
Continue taking your medicine for as long as your doctor tells you to.
This medicine helps to treat your condition but it does not cure it. It is important to keep taking your medicine even if you feel well.
Your doctor may want to gradually reduce the amount of NOTEN you are taking. This should take place over a period of approximately two weeks before stopping it completely.
Do not stop taking this medicine suddenly as this may worsen your condition.
If you forget to take it
If it is less than six hours from when you missed your dose, take it as soon as you remember, and then go back to taking your tablets at the same time you would normally
If it is more than six hours since your last dose, skip the dose you missed and take your next dose when you would normally.
Do not take a double dose to make up for the dose you missed.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or Poisons Information Centre (telephone 13 11 26), or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much NOTEN. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
If you take too much NOTEN, you may feel faint, dizzy or lightheaded, wheeze or have difficulty breathing. You may also have a very slow heartbeat.
While you are taking NOTEN
Things you must do
If you are about to be started on any new medicine, tell your doctor and pharmacist that you are taking NOTEN.
Tell any other doctors, dentists and pharmacists who treat you that you are taking this medicine.
If you become pregnant while taking this medicine, tell your doctor immediately.
If you have an allergic reaction to any foods, medicines or insect stings, tell your doctor immediately. NOTEN can cause allergic reactions to be worse and harder to treat.
If you are going to have surgery, tell your surgeon or dentist that you are taking this medicine. It may affect other the medicines used during surgery.
If you have diabetes, check your blood sugar level regularly and report any changes to your doctor. NOTEN may affect your diabetes. It may hide the symptoms of low blood sugar levels, such as a fast heartbeat. It may also take longer for your low blood sugar to get back to normal even if you follow the usual treatment for diabetes. Your doctor may need to change your dose of diabetic medicines, including insulin.
If you have angina and continue to have angina attacks or have more of them while you are taking this medicine, tell your doctor. NOTEN is used to help prevent angina, so your angina attacks should become less severe and occur less often.
If you feel light-headed, dizzy or faint when getting out of bed or standing up, get up slowly. Standing up slowly, especially when you get up from bed or chairs, will help your body get used to the change in position and blood pressure.
If this problem gets worse or continues, talk to your doctor.
Make sure you drink enough water in hot weather and during exercise while you are taking NOTEN, especially if you sweat a lot. If you do not drink enough water you may feel faint, lightheaded or sick. The recommended healthy minimum water intake is 6 to 8 glasses a day.
If you have to have any medical tests, tell your doctor that you are taking this medicine.
Keep all of your doctor's appointments so that your progress can be checked.
Things you must not do
Do not take NOTEN to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop taking your medicine or lower the dose without checking with your doctor. If you stop taking it suddenly, your condition may worsen or other heart complications may occur.
Do not take any new medicines with NOTEN unless your doctor has told you to.
Things to be careful of
Be careful driving or operating machinery until you know how NOTEN affects you. NOTEN may cause dizziness, tiredness, light headedness or faintness in some people. If you have any of these symptoms, do not drive, operate machinery or do anything else that could be dangerous.
Be careful not to over exercise when you first start taking NOTEN. This medicine helps to prevent angina resulting from physical activity and exercise. Talk to your doctor about how much exercise you can do.
Dress warmly during cold weather, especially if you will be outside for a long time (for example, when playing or watching sports in winter). NOTEN, like other beta-blocker medicines, may make you more sensitive to cold temperatures, especially if you have blood circulation problems. Beta-blockers tend to decrease blood circulation to the skin, fingers and toes.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking NOTEN.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
If you get any side effects, do not stop taking NOTEN without first talking to your doctor.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- stomach upsets such as diarrhoea, constipation, abdominal pain or heartburn (indigestion)
- dry mouth, change in taste sensation
- dizziness, headache or buzzing or ringing in the ears
- slow or irregular heart beat
- dry eyes, problems with vision
- runny or blocked nose
- difficulty sleeping, nightmares, vivid dreams
- skin reactions (e.g. rash, itching, worsening of psoriasis
- increased hair loss
- tingling, 'pins and needles' or walking unsteadily
- sexual problems
These side effects are usually mild.
Tell your doctor as soon as possible if you notice any of the following:
- confusion or disorientation
- depression or mood changes or a worsening of these
- unusual thoughts, hallucinations (seeing, feeling or hearing things that are not there)
- light headedness or fainting, which may be due to low blood pressure
- yellowing of the skin and/or eyes (jaundice)
The above list includes serious side effects that may require medical attention. Serious side effects are rare.
If any of the following happen, tell your doctor immediately or go to Accident and Emergency at your nearest hospital:
- wheezing, chest tightness or difficulty breathing
- unusual bruising or bleeding
- fast, slow or irregular heart beat
- swelling of the face, lips, tongue or other parts of the body
The above list includes very serious side effects. You may need urgent medical attention or hospitalisation. These side effects are rare.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Other side effects not listed above may also occur in some people.
After using NOTEN
Storage
Keep your tablets in the pack until it is time to take them. If you take the tablets out of the pack they may not keep well.
Keep your tablets in a cool dry place where the temperature stays below 25°C.
Do not store NOTEN or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine, or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
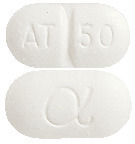
NOTEN is a white, oblong tablet marked with AT/50 and a Greek alpha symbol.
Each pack contains 30 tablets.
Ingredients
NOTEN contains 50 mg of atenolol as the active ingredient.
The tablets also contain:
- lactose monohydrate
- maize starch
- povidone
- microcrystalline cellulose
- colloidal anhydrous silica
- hydrogenated vegetable oil
- crospovidone
- sodium starch glycollate
- purified talc
- magnesium stearate.
NOTEN contains sulfites and sugars as lactose monohydrate. This medicine does not contain sucrose, gluten, tartrazine or any other azo dyes.
Manufacturer
Alphapharm Pty Ltd
Level 1, 30 The Bond
30 - 34 Hickson Road
Millers Point NSW 2000
www.mylan.com.au
This leaflet was prepared in June 2021.
Australian registration numbers:
AUST R 46250 (blister pack)
noten_cmi\Jun21/00
Published by MIMS August 2021

 Chemical name: 2-[4-[(2RS)-2-hydroxy-3-[(1-methylethyl)amino]propoxy]phenyl]acetamide.
Chemical name: 2-[4-[(2RS)-2-hydroxy-3-[(1-methylethyl)amino]propoxy]phenyl]acetamide.