What is in this leaflet
This leaflet answers some common questions about PRAMIN.
It does not contain all of the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have benefits and risks. Your doctor has weighed the risks of you taking PRAMIN against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, talk to your doctor or pharmacist.
Keep this leaflet with your medicine. You may need to read it again.
What PRAMIN is used for
In Adults over 20 years, PRAMIN is used to:
- control nausea and vomiting associated with using other medicines, kidney disease, radiation or chemotherapy treatment, cancer, childbirth, infectious diseases, or surgery
- in the management of certain stomach problems associated with diabetes
- with X-ray examinations of the stomach and/or intestines
- activate stomach contractions in conditions where there is a need to encourage normal passage of food through the stomach and intestines
- help with passing tubes into the intestines
In young adults and children over 1 year of age this medicine is used to
- treat severe vomiting of known cause or following chemotherapy or radiation treatment
- help with passing tubes into the intestine
PRAMIN belongs to a group of medicines called anti-emetics and is thought to work by blocking the action of a chemical in the brain which causes nausea and vomiting. It also increases the muscle contractions in the stomach and small intestine.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
This medicine is available only with a doctor's prescription.
There is no evidence that PRAMIN is addictive.
Before you take PRAMIN
When you must not take it
Do not take PRAMIN if you are allergic to medicines containing metoclopramide hydrochloride or any of the ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include:
- wheezing or shortness of breath
- swelling of the face, lips or tongue which may cause difficulty in swallowing or breathing
- skin rash, itching or hives on the skin
Do not take PRAMIN if you have any of the following:
- active bleeding from the stomach and/or digestive tract
- blockage of the stomach and/or digestive tract
- recent surgery of the stomach and/or digestive tract
- phaeochromocytoma (an adrenaline-producing tumour of the adrenal gland)
- epilepsy (fits or convulsions)
- take other medicines likely to cause extrapyramidal effects, such as antipsychotics/neuroleptics and certain antidepressants that can cause movement disorders. This reaction may include trembling and a sudden onset of uncontrollable muscle spasms affecting the eyes, head, neck and body.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, dyes or preservatives.
Tell your doctor if you are pregnant or plan to become pregnant. Your doctor will discuss the risks and benefits of taking this medicine during pregnancy.
Tell your doctor if you are breastfeeding or wish to breastfeed. PRAMIN passes into breast milk and can affect the flow of your breast milk. Your doctor will discuss the risks and benefits of taking this medicine when breastfeeding.
Tell your doctor if you have or have had any of the following medical conditions:
- breast cancer
- liver or kidney problems
- Parkinson's disease - a condition affecting muscle control and movement
- depression
high blood pressure
Your doctor may want to take special care if you have any of these conditions.
If you have not told your doctor about any of the above, tell him/her before you start taking PRAMIN.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you buy without a prescription from a pharmacy, supermarket or health food shop.
Some medicines and PRAMIN may interfere with each other. These include:
- certain medicines used to treat mental disorders, including lithium, thioridazine
- pain relievers such as codeine, morphine and paracetamol
- atropine-like medicines used to prevent travel sickness and for stomach cramps
- medicines used to relieve anxiety and/or help you sleep
- levodopa, a medicine used in the treatment of Parkinson's disease
- digoxin, a medicine used to treat heart failure
- tetracycline, an antibiotic
- ciclosporin, a medicine used to help prevent organ transplant rejection
- monoamine oxidase inhibitors, a group of medicines used to treat depression.
These medicines may be affected by PRAMIN or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
How to take PRAMIN
Follow all directions given to you by your doctor and pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the pack, ask your doctor or pharmacist for help.
How much to take
Your doctor may advise you to take a different dose. This depends on your condition and whether or not you are taking any other medicines.
The dose varies with the age of the patient and reason for use.
The total daily dosage of PRAMIN, especially for children and young adults, should not normally exceed 0.5mg/kg bodyweight or 30mg daily.
Space the doses as evenly as possible throughout the day.
20 + years: 1 tablet every 8 hours
15 to 20 years: ½ to 1 tablet every 8 hours
Children and young adults are very sensitive to the effects of PRAMIN. Your doctor will normally start the treatment at the lower dose. Do not exceed the prescribed dose in these age groups.
How to take it
Swallow the tablets with a full glass of water.
The tablets can be broken in half (along the break line).
Do not exceed the prescribed dose.
When to take it
PRAMIN is best taken 30 minutes before symptoms are likely to occur or 30 minutes before meals. Space the doses of PRAMIN evenly throughout the day.
Your doctor may tell you to take PRAMIN only when required for each occasion of nausea or vomiting.
How long to take it for
Continue taking your medicine for as long as your doctor tells you.
If you forget to take it
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take the missed dose as soon as you remember, and then go back to taking your tablets as you would normally.
Do not take a double dose to make up for the dose you missed. This may increase the chance of getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital, if you think you or anyone else may have taken too much PRAMIN. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
If you take too much PRAMIN, you may experience drowsiness, dizziness, agitation, headache, nausea, vomiting, and unusual movements, such as trembling and shaking of the hands and feet, and uncontrolled movements of the tongue, mouth or jaw.
While you are taking PRAMIN
Things you must do
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking PRAMIN.
Tell any other doctors, dentists and pharmacists who treat you that you are taking this medicine.
If you plan to have surgery, including dental surgery, tell your surgeon, anaesthetist or dentist that you are taking PRAMIN.
If you become pregnant while taking this medicine, tell your doctor immediately.
If you need to have any liver function tests or other tests, tell your doctor. PRAMIN may affect the results of some tests.
If your symptoms do not improve or if they become worse, tell your doctor.
Things you must not do
Do not use PRAMIN to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Things to be careful of
Be careful driving or operating machinery until you know how PRAMIN affects you. PRAMIN may cause drowsiness, dizziness or lightheadedness in some people. If any of these occur, do not drive, operate machinery or do anything else that could be dangerous. Children should be careful when riding bicycles or climbing trees.
Be careful when drinking alcohol while taking PRAMIN. Combining PRAMIN and alcohol can make you more sleepy.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking PRAMIN.
Like all other medicines, PRAMIN may have unwanted side effects in some people. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
If you are over 65 years of age, you may have an increased chance of getting side effects.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- drowsiness
- fatigue, tiredness
- anxiety, restlessness, agitation
- trouble sleeping
- diarrhoea, constipation, bowel irregularities
- headache, dizziness
- breast enlargement, unusual secretion of breast milk.
The above list includes the more common or mild side effects of PRAMIN.
Tell your doctor as soon as possible if you notice any of the following:
- uncontrolled and repeated movements of the face, jaw or tongue, arms or legs. This may be a sign of tardive dyskinesia, a movement disorder which can be potentially irreversible
- yellowing of the skin or eyes
- fast or irregular heart beat
- swelling of hands, ankles or feet.
The above list includes serious side effects which may require medical attention. Serious side effects are rare.
If any of the following happen, stop taking PRAMIN and tell your doctor immediately, or go to Accident and Emergency at the nearest hospital:
- symptoms of an allergic reaction such as, skin rash, itching or hives; swelling of the face, lips or tongue which may cause difficulty in swallowing or breathing; wheezing or shortness of breath
- sudden uncontrolled muscle spasms, stiffness of the arms or legs, muscle spasms of the face, locked jaw or upturned eyes
- trembling of the hands or legs, slowing of all movements, shuffling walk
- a sudden increase in body temperature, extremely high blood pressure, stiff muscles and severe convulsions. These could be signs of a serious side effect called neuroleptic malignant syndrome.
These are rare yet serious side effects and may need urgent medical attention.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Other side effects not listed above may also occur in some patients.
After taking PRAMIN
Storage
Keep your tablets in the bottle or blister pack until it is time to take them. If you take the tablets out of the bottle or blister pack they may not keep well.
Keep your tablets in a cool dry place where the temperature stays below 30°C.
Do not store PRAMIN or any other medicine in the bathroom or near a sink. Do not leave it in the car or on a window sill. Heat and dampness can destroy some medicines.
Keep PRAMIN where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
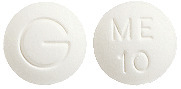
PRAMIN is a white, normal convex tablet marked "ME" over "10" on one side and "G" on the reverse.
Available in bottles of 100 tablets or blister packs of 25 tablets.
Ingredients
The active ingredient in PRAMIN is metoclopramide hydrochloride monohydrate. Each PRAMIN tablet contains 10 mg of metoclopramide hydrochloride.
The tablets also contain:
- lactose anhydrous
- pregelatinised maize starch
- microcrystalline cellulose
- colloidal anhydrous silica
- magnesium stearate.
PRAMIN tablets contain sugars as lactose and trace amounts of sulfites.
Supplier
PRAMIN is supplied in Australia by:
Alphapharm Pty Ltd trading as Viatris
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.viatris.com.au
Phone: 1800 274 276
Australian registration numbers:
PRAMIN bottle - AUST R 17661
PRAMIN blister - AUST R 364450
This leaflet was prepared in November 2023.
PRAMIN_cmi\Nov23/00
Published by MIMS January 2024

 Molecular formula: C14H22ClN3O2,HCl,H2O. Molecular weight: 354.3.
Molecular formula: C14H22ClN3O2,HCl,H2O. Molecular weight: 354.3.