WHAT IS IN THIS LEAFLET
This leaflet answers some common questions about Sertraline Sandoz.
It does not contain all the available information.
It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking this medicine against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
WHAT SERTRALINE SANDOZ IS USED FOR
This medicine is used to treat depression and conditions called obsessive compulsive disorder (OCD) and panic disorder.
It contains the active ingredient sertraline hydrochloride.
Sertraline hydrochloride belongs to a group of medicines called selective serotonin reuptake inhibitors (SSRIs).
It works by blocking the uptake of a chemical called serotonin into nerve cells in the brain. Serotonin and other chemicals called amines are involved in controlling mood.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
Sertraline Sandoz should not be used in children and adolescents under the age of 18 years for the treatment of any medical condition other than obsessive compulsive disorder (OCD). The safety and efficacy of Sertraline Sandoz for the treatment of medical conditions (other than OCD) in this age group has not been satisfactorily established.
For the treatment of OCD, Sertraline Sandoz is not recommended for use in children under the age of 6, as the safety and efficacy in children of this age group has not been established.
This medicine is only available with a doctor's prescription.
There is no evidence that Sertraline Sandoz is addictive.
BEFORE YOU TAKE SERTRALINE SANDOZ
When you must not take it
Do not take this medicine if you have an allergy to:
- sertraline hydrochloride, the active ingredient, or to any of the other ingredients listed at the end of this leaflet under Product description
- any other similar medicines such as other medicines in the same class (SSRIs).
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin.
Do not take this medicine if you have epilepsy not properly controlled by medication.
Do not take this medicine if you are taking another medicine for depression called a monoamine oxidase inhibitor (MAOI) or have been taking it within the last 14 days. Taking Sertraline Sandoz with a MAOI such as moclobemide, selegiline, phenelzine and tranylcypromine may cause a serious reaction with a sudden increase in body temperature, extremely high blood pressure and convulsions (fits).
Do not take this medicine if you are taking:
- tryptophan (contained in protein-based foods or dietary proteins)
- methadone (used to treat drug addiction)
- dextromethorphan (used as a cough suppressant in cold and flu medications)
- medicines used for pain management e.g. fentanyl, tapentadol, tramadol or pethidine
- medicines used to treat migraine e.g. sumatriptan
- phentermine, used to help weight loss.
These medicines can cause an exaggerated response to Sertraline Sandoz.
Do not take this medicine if you are taking pimozide, used to treat disturbances in thinking, feeling and behaviour.
Ask your doctor or pharmacist if you are not sure if you have been taking one of these medicines.
Do not give Sertraline Sandoz to children or adolescents under the age of 18 unless the doctor has prescribed it for the treatment of OCD. Sertraline Sandoz is not suitable for children under 6 years of age.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you are pregnant or plan to become pregnant. There have been reports that babies exposed to sertraline hydrochloride and other antidepressants during the third trimester of pregnancy may develop complications after birth.
Tell your doctor if you are breastfeeding or wish to breastfeed. Sertraline Sandoz passes into breast milk and may affect your baby.
Your doctor will discuss the risks and benefits of using this medicine when pregnant or breastfeeding.
Tell your doctor if you have or have had any of the following medical conditions:
- epilepsy or seizures (fits)
- liver or kidney problems
- any other mental illness
- a tendency to bleed more than normal
- heart conditions causing irregular heartbeats
- diabetes mellitus
- glaucoma, an eye condition.
If you have not told your doctor about any of the above, tell him/ her before you start taking Sertraline Sandoz.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including all prescription medicines, any medicines, vitamins, herbal supplements or natural therapies that you get without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and Sertraline Sandoz may interfere with each other. These include:
- monoamine oxidase inhibitors (MAOIs), medicines used for the treatment of depression.
Taking Sertraline Sandoz with, or within 14 days of stopping a MAOI may cause a serious reaction with a sudden increase in body temperature, extremely high blood pressure and convulsions. - other MAOI drugs such as linezolid, an antibiotic used to treat pneumonia and certain skin infections
- other medicines used for depression, panic disorder, social anxiety disorder, obsessive illnesses (e.g. fluoxetine, paroxetine, fluvoxamine, citalopram, venlafaxine)
- lithium, a medicine used to treat mood swings
- tryptophan (contained in protein-based foods or dietary proteins)
- phentermine (weight-reducing medicines)
- dextromethorphan, used in cold and flu medicines to suppress cough
- medicines for strong pain management such as fentanyl, tapentadol, tramadol or pethidine
- methadone, a medicine used to treat drug addiction
- medicines used to relieve pain, swelling and other symptoms of inflammation, including arthritis (e.g. aspirin or NSAIDs such as ibuprofen or diclofenac)
- pimozide, used to treat disturbances in thinking, feeling and behaviour
- St John's wort, a herbal remedy used to treat mood disorders
- clozapine, a medicine used to treat schizophrenia
- medicines used to treat Attention Deficit Hyperactivity Disorder (ADHD) such as dexamphetamine and lisdexamphetamine
- diazepam or other medicines that act on the brain or nervous system
- phenytoin, a medicine used to treat epilepsy
- sumatriptan, a medicine used to treat migraine
- medicines for irregular heartbeats e.g. flecainide
- warfarin or other medicines that stop the blood from clotting
- cimetidine, a medicine used to treat reflux and ulcers
- antibiotics
- grapefruit.
These medicines may be affected by Sertraline Sandoz, or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
Your doctor or pharmacist has more information on medicines to be careful with or avoid while taking this medicine.
HOW TO TAKE SERTRALINE SANDOZ
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions, ask your doctor or pharmacist for help.
How much to take
For depression in adults, the usual starting dose is one Sertraline Sandoz 50 mg tablet each day. The dose can be increased gradually up to a maximum dose of 200 mg a day if necessary.
For obsessive compulsive disorder in children (6-12 years) the usual starting dose is 25 mg per day (half a 50 mg Sertraline Sandoz tablet), increasing to 50 mg per day after one week.
For obsessive compulsive disorder in adults and adolescents (13-18 years) the usual starting dose is one 50 mg Sertraline Sandoz tablet each day.
For panic disorder in adults the usual starting dose is 25 mg per day (half a 50 mg Sertraline Sandoz tablet), increasing to 50 mg per day after one week.
The maximum recommended dose of Sertraline Sandoz for the conditions listed above is 200 mg per day.
Ask your doctor or pharmacist if you are unsure of the correct dose for you. They will tell you exactly how much to take.
How to take it
Swallow the tablet(s) with a full glass of water.
The tablets can be broken in half, but should not be chewed.
If you need to break Sertraline Sandoz hold the tablet with both hands and snap along the break-line.
When to take Sertraline Sandoz
Take your medicine at about the same time each day, either in the morning or evening. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
Sertraline Sandoz can be taken with or without food.
How long to take Sertraline Sandoz
Continue taking your medicine for as long as your doctor tells you.
Most medicines for depression take time to work, so do not be discouraged if you do not feel better straight away.
It may take 2 to 4 weeks or even longer to feel the full benefit of this medicine.
Even when you feel well, you may need to take Sertraline Sandoz for several months or longer.
Continue taking this medicine until your doctor tells you to stop.
Do not stop taking Sertraline Sandoz, or change the dose, without first checking with your doctor.
Occasionally the symptoms of depression or other psychiatric conditions may include thoughts of harming yourself or committing suicide. It is possible that these symptoms may continue or increase until the full anti-depressant effect of your medicine becomes apparent (i.e. one to two months).
You or anyone close to you or caring for you should watch for these symptoms and tell your doctor immediately or go to the nearest hospital if you have any distressing thoughts or experiences during this initial period or at any other time.
Contact your doctor if you experience any worsening of your depression or other symptoms at any time during your treatment.
If you forget to take it
Take your dose as soon as you remember, and continue to take it as you would normally.
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Do not take a double dose to make up for the dose you missed. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering when to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26 or New Zealand 0800 POISON or 0800 764766) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else has taken too much Sertraline Sandoz. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
If you take too many tablets you may feel drowsy, sick in the stomach (nausea, vomiting or diarrhoea), have fast or irregular heartbeats, suffer from tremors, feel agitated or dizzy. Coma has also been reported with overdose.
WHILE YOU ARE TAKING SERTRALINE SANDOZ
Things you must do
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking Sertraline Sandoz.
Tell any other doctors, dentists and pharmacists who treat you that you are taking this medicine.
If you become pregnant while taking this medicine, tell your doctor immediately.
If you are a woman of child-bearing age, you should avoid becoming pregnant while taking Sertraline Sandoz.
Tell your doctor immediately if you have any suicidal thoughts or other mental/mood changes. A worsening of depressive symptoms including thoughts of suicide or self-harm may occur in the first one or two months of you taking this medicine or when the doctor changes your dose. These symptoms should be controlled when the full effect of Sertraline Sandoz takes place.
Children, adolescents or young adults under the age of 24 years are more likely to experience these effects during the first few months of treatment.
Patients and caregivers should be alert and monitor for these effects.
Signs and symptoms of suicide include:
- thoughts or talk of death or suicide
- thoughts or talk of self-harm or harm to others
- any recent attempts of self-harm
- increase in aggressive behaviour, irritability or agitation
- worsening of depression.
All mention of suicide or violence must be taken seriously.
If you or someone you know is demonstrating these warning signs of suicide while taking Sertraline Sandoz, contact your doctor or a mental health professional right away.
Children should have regular check-ups with the doctor to monitor growth and development.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking this medicine. It may interact with other medicines used during surgery and cause unwanted side effects.
If you are about to have any urine tests, tell your doctor that you are taking this medicine. It may interfere with the results of some tests.
Things you must not do
Do not take Sertraline Sandoz to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop taking your medicine or change the dose without checking with your doctor.
Do not let yourself run out of tablets over the weekend or on holidays.
Suddenly stopping Sertraline Sandoz can cause dizziness, light headedness, numbness, unusual tingling feelings or shakiness.
Things to be careful of
Be careful driving or operating machinery until you know how Sertraline Sandoz affects you. Some medicines for depression may affect your ability to drive or operate machinery or do things that could be dangerous if you are not alert.
Although drinking moderate amounts of alcohol is unlikely to affect your response to Sertraline Sandoz, your doctor may suggest avoiding alcohol while you are taking this medicine.
If you are feeling drowsy or are uncoordinated, be careful that you do not fall over. Sertraline Sandoz, like other medicines in this class, may increase your risk of bone fracture.
You should wait at least 14 days after stopping Sertraline Sandoz before starting medicines for depression or obsessive illnesses from the MAOI medicine group such as moclobemide, selegiline, phenelzine and tranylcypromine.
All of the above precautions are important even after you have stopped taking Sertraline Sandoz.
The effects of Sertraline Sandoz may last for some days after you have stopped taking it.
SIDE EFFECTS
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Sertraline Sandoz.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
It can be difficult to tell whether side effects are the result of taking Sertraline Sandoz, effects of your condition or side effects of other medicines you may be taking. For this reason it is important to tell your doctor of any change in your condition.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- headache, dizziness, shaking or tremors, unusually overactive, muscle stiffness or weakness, decrease or loss of touch or other senses, sleepiness, drowsiness, impaired concentration
- dry mouth, nausea, feeling sick, diarrhoea, indigestion, vomiting, stomach pain, constipation
- increased sweating, rash and hives
- tiredness, fever, feeling unwell
- hot flush, high blood pressure
- weight increase or loss
- increased or decreased appetite
- sleeping difficulties
- vision disturbance
- menstrual irregularities, sexual problems, sexual dysfunction including impaired sexual function in males, difficulty in passing urine, blood in the urine, or increased frequency
- unusual hair loss or thinning
- increased sensitivity of the skin to sun
- persistent noise in the ears
- tingling and numbness of hands and feet
- inflammation of the colon (causing diarrhoea).
Tell your doctor as soon as possible if you notice any of the following:
- agitation, nervousness, anxiety, frightening dreams, yawning, abnormal thinking, teeth grinding, symptoms of agitation, anxiety, dizziness, headache, nausea and tingling or numbness of the hands and feet after stopping Sertraline Sandoz
- uncontrollable muscle spasms affecting the eyes, head, neck and body, temporary paralysis or weakness of muscles
- lockjaw
- painful, swollen joints
- difficulty in breathing, wheezing or coughing
- uncontrollable movements of the body, shuffling walk, unusual weakness
- palpitations, fainting or chest pain
- irregular heartbeats
- abnormal bleeding including vaginal bleeding
- sudden onset of severe headache.
If any of the following happen tell your doctor immediately or go to Accident and Emergency at your nearest hospital:
- fits or seizures
- signs of allergy such as rash or hives, swelling of the face, lips or tongue, wheezing or difficulty breathing
- symptoms of sudden fever with sweating, fast heart beat and muscle stiffness, which may lead to loss of consciousness
- severe blisters and bleeding in the lips, eyes, mouth, nose and genitals
- fever, sore throat, swollen glands, mouth ulcers, unusual bleeding or bruising under the skin.
- thoughts of suicide or attempting suicide or self-harm.
The following symptoms are signs of side effects named Serotonin Syndrome (SS) or Neuroleptic Malignant Syndrome (NMS). SS is caused by edications which build up high levels of serotonin in the body. NMS is a life threatening emergency associated with the use of antipsychotic medicines. The risk of SS and NMS with SSRI’s is increased with combined use of other SSRIs, MAOIs and other antipsychotic medicines.
- agitation
- hallucinations
- coma
- fast heart beat
- fluctuating blood pressure readings
- high body temperature
- twitching and spastic body movements
- lack of body co-ordination
- nausea
- vomiting
- diarrhoea
The above list includes very serious side effects. You may need urgent medical attention or hospitalisation.
Tell your doctor if you notice anything else that is making you feel unwell. Other side effects not listed above may also occur in some people. Some of these side effects (e.g., changes in thyroid function, liver function or glucose control) can only be found when your doctor does tests from time to time to check your progress.
AFTER TAKING SERTRALINE SANDOZ
Storage
Keep your medicine in the original container.
If you take it out of its original container it may not keep well.
Keep your medicine in a cool dry place where the temperature stays below 25°C.
Do not store Sertraline Sandoz or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car.
Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
PRODUCT DESCRIPTION
What it looks like
Sertraline Sandoz comes in two types of tablets:
Sertraline Sandoz 50 mg - white, capsule shaped, scored, film-coated tablet, marked with SE|50 on one side.
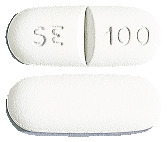
Sertraline Sandoz 100 mg - white, capsule shaped, scored, film-coated tablet, marked with SE|100 on one side.
Available in blisters of 30 tablets.
Not all strengths may be available in Australia.
Ingredients
Active ingredients:
- Sertraline Sandoz 50 mg – 50 mg sertraline (as hydrochloride)
- Sertraline Sandoz 100 mg – 100 mg sertraline (as hydrochloride).
Inactive ingredients:
- microcrystalline cellulose
- calcium hydrogen phosphate dihydrate
- hyprolose
- sodium starch glycollate
- magnesium stearate
- hypromellose
- purified talc
- titanium dioxide.
This medicine does not contain lactose, sucrose, gluten, tartrazine or any other azo dyes.
Supplier
Sandoz Pty Ltd
ABN 60 075 449 553
54 Waterloo Road
Macquarie Park, NSW 2113
Australia
Tel: 1800 726 369
Novartis New Zealand Ltd
PO Box 99102
Newmarket, Auckland 1149
New Zealand
Tel: 0800 354 335
This leaflet was revised in March 2023.
Australian Register Numbers
50 mg tablets: AUST R 98697 (blisters)
100 mg tablets: AUST R 98698 (blisters)
Published by MIMS May 2023


 Chemical name: (1S,4S)-4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-N-methyl-1-naphthalenamine hydrochloride.
Chemical name: (1S,4S)-4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-N-methyl-1-naphthalenamine hydrochloride.