What is in this leaflet
This leaflet answers some common questions about VEXAZONE.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have benefits and risks. Your doctor has weighed the risks of you taking VEXAZONE against the benefits expected for you.
If you have any concerns about taking this medicine, talk to your doctor or pharmacist.
Keep this leaflet with your medicine. You may need to read it again.
What VEXAZONE is used for
VEXAZONE is a tablet that is used to improve the action of the body's naturally produced insulin. VEXAZONE is used to treat type 2 diabetes mellitus not adequately controlled by diet and exercise.
Type 2 diabetes is also known as non-insulin dependent diabetes mellitus or adult-onset diabetes, and is controlled by diet, exercise, certain oral medications and occasionally insulin.
This medicine is also called pioglitazone and is a member of a class of drugs called glitazones. They are insulin-sensitising agents, which decrease insulin resistance. These medicines help control the level of sugar in your blood when you have type 2 diabetes by helping your body make better use of the insulin it produces.
VEXAZONE may be used on its own (when diet and exercise is not enough to treat your diabetes) or in combination with metformin and/or a sulphonylurea which are also oral anti-diabetic medicines.
It may also be used in combination with insulin.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
This medicine is available only with a doctor's prescription.
The use of VEXAZONE has not been studies in children.
Before you take VEXAZONE
When you must not take it
Do not take VEXAZONE if:
- you have heart failure requiring treatment. Talk to your doctor if you have heart failure
- type 1 diabetes or diabetic ketoacidosis (often caused by very high blood glucose levels)
- you have an allergy to any medicine containing pioglitazone hydrochloride or any of the ingredients listed at the end of this leaflet (see ‘Product Description’)
Some of the symptoms of an allergic reaction may include shortness of breath; wheezing or difficulty breathing; swelling of the face, lips, tongue or other parts of the body; rash, itching or hives on the skin.
Do not take VEXAZONE if you have heart failure requiring treatment.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you have or have had any of the following medical conditions:
- heart disease with shortness of breath after minimal physical activity
- heart disease with severe symptoms at rest
- swelling of hands, ankles or feet
- bladder cancer or symptoms associated with bladder cancer such as blood in the urine (hematuria) often accompanied by pain and burning
- liver problems
- kidney problems that require dialysis. VEXAZONE is not recommended for use if you are on dialysis
Talk to your doctor about the risk of fracture and for advice on how to keep your bones healthy. Bone fractures, usually in the hand, upper arm or foot, have been seen in some women when taking VEXAZONE.
Tell your doctor if you are pregnant or plan to become pregnant. Like most medicines, VEXAZONE is not recommended for use during pregnancy. If there is a need to consider VEXAZONE during your pregnancy, your doctor will discuss with you the benefits and risks of taking VEXAZONE.
Tell your doctor if you are breastfeeding or plan to breastfeed. It is not known whether VEXAZONE passes into breast milk. Therefore, it is recommended to not breast-feed while taking this medicine.
Talk to your doctor if you are a woman who has not reached menopause, but have no menstrual periods. Some women who do not have monthly periods and have not been through menopause may restart their periods when taking VEXAZONE. These women may be at increased risk of pregnancy.
Tell your doctor if you are using another medicine for diabetes. VEXAZONE can enhance the action of other medicines. You may be at risk of low blood sugar (hypoglycaemia). If this happens, your doctor may need to adjust the dose of your other medicines.
Tell your doctor if you suffer from lactose intolerance. VEXAZONE tablets contain lactose monohydrate.
If you have not told your doctor about any of the above, tell him/her before you start taking VEXAZONE.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from a pharmacy, supermarket or health food shop.
Some medicines and VEXAZONE may interfere with each other. These include:
- chlorpropamide
- glibenclamide
- gliclazide
- insulin
- metformin
- oral contraceptives
- gemfibrozil
- rifampicin
- tolbutamide
These medicines may be affected by VEXAZONE or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
Your doctor or pharmacist has more information on medicines to be careful with or to avoid while taking this medicine.
How to take VEXAZONE
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the pack, ask your doctor or pharmacist for help.
How much to take
The dose varies from person to person. Your doctor will decide the right dose for you.
The dose your doctor will prescribe for you will usually be in the range of 15 mg to 45 mg per day.
How to take it
Swallow the tablet whole with a glass of water.
When to take it
VEXAZONE must be taken once daily, at about the same time each day. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
It does not matter if you take this medicine before or after food.
How long to take it
Continue taking your medicine for as long as your doctor tells you to. This medicine helps to control your condition, but does not cure it. It is important to keep taking your medicine even if you feel well.
If you forget to take it
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take it as soon as you remember, and then go back to your medicine as you would normally.
Do not take a double dose to make up for the dose you missed.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much VEXAZONE. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
While you are taking VEXAZONE
Things you must do
It is important that you remember to take VEXAZONE daily and at the dose prescribed by your doctor.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking VEXAZONE.
Tell any other doctors, dentists and pharmacists who treat you that you are taking this medicine.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking this medicine. VEXAZONE may affect other medicines used during surgery.
If you become pregnant while taking this medicine, tell your doctor immediately.
If you are about to have any blood tests, tell your doctor that you are taking this medicine. It may interfere with the results of some tests.
Keep all of your doctor's appointments so that your progress can be checked. Your doctor may do some tests from time to time to make sure the medicine is working and to prevent unwanted side effects.
Tell your doctor if you have gained weight since taking this medicine. Weight gain can be associated with improved blood sugar control; however, it may also be a symptom of heart failure.
Things you must not do
Do not take VEXAZONE to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop taking your medicine or lower the dosage without checking with your doctor. If you stop taking it suddenly, your condition may worsen or you may have unwanted side effects.
Things to be careful of
VEXAZONE alone is unlikely to affect your ability to drive or operate machinery. However, be careful not to let your blood glucose levels fall too low whilst driving or operating machinery if using VEXAZONE in combination with other anti-diabetic medicines. If your blood glucose level becomes too low, you may feel dizzy, light headed, weak or tired and your reaction time may be slower than usual.
If you have any of these symptoms, do not drive or do anything else that could be dangerous.
Lifestyle measures that help reduce heart disease risk
By following these simple measures, you can further reduce the risk from heart disease.
- Quit smoking and avoid second-hand smoke.
- Limit alcohol intake.
- Enjoy healthy eating by:
- eating plenty of vegetables and fruit;
- reducing your saturated fat intake (eat less fatty meats, full fat dairy products, butter, coconut and palm oils, most take-away foods, commercially-baked products). - Be active. Progress, over time, to at least 30 minutes of moderate-intensity physical activity on 5 or more days each week. Can be accumulated in shorter bouts of 10 minutes duration. If you have been prescribed anti-angina medicine, carry it with you when being physically active.
- Maintain a healthy weight.
- Discuss your lifestyle and lifestyle plans with your doctor.
- For more information and tools to improve your heart health, call Heartline, the Heart Foundation's national telephone information service, on 1300 36 27 87 (local call cost).
Know warning signs of heart attack and what to do:
- Tightness, fullness, pressure, squeezing, heaviness or pain in your chest, neck, jaw, throat, shoulders, arms or back.
- You may also have difficulty breathing, or have a cold sweat or feel dizzy or light headed or feel like vomiting (or actually vomit).
- If you have heart attack warning signs that are severe, get worse or last for 10 minutes even if they are mild, call triple zero (000). Every minute counts.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking VEXAZONE. This medicine helps most people with type 2 diabetes not controlled by diet, but it may have unwanted side effects in some people.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Some side effects may be related to the dose of VEXAZONE. Accordingly, it is important that you tell your doctor as soon as possible about any unwanted effects. Your doctor may then decide to adjust the dose of VEXAZONE you are taking.
Do not be alarmed by the following list of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
A few patients have experienced the following side effects whilst taking VEXAZONE:
- hypoglycaemia (low blood sugar). This occurs more often when VEXAZONE is taken with a sulfonylurea or insulin
- shortness of breath when at rest or after minimal physical activity with swelling of the legs, feet and hands, rapid increase in weight
- a small increase in weight
- heart failure which may show as localised swelling of the ankles, feet and hands (oedema) and/or fluid in the lungs (pulmonary oedema). This has been reported in clinical trials mainly in patients who are taking VEXAZONE in combination with insulin
- increased risk of fracture in women
- macular oedema (an eye disorder that can affect vision)
- altered or impaired liver function.
Tell your doctor if you notice any of the following:
- weight gain
- signs of hypoglycaemia (low blood sugar), which may include sweating, weakness, hunger, dizziness, trembling or shaking, light-headedness, headache, lack of concentration, tearfulness or crying, irritability, numbness around the lips and fingers
- eye problems including blurred or double vision.
The above list includes the more common side effects of your medicine.
Tell your doctor immediately if you notice any of the following:
- dark urine or pale stools, yellowing of the skin or eyes, severe cramps of the stomach, nausea or vomiting, loss of weight, tiredness
- blood in the urine often accompanied by pain and burning, these can be symptoms of bladder cancer.
The above list includes very serious side effects. You may need urgent medical attention. Serious side effects are rare.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Other side effects not listed above may also occur in some people.
After taking VEXAZONE
Storage
Keep your tablets in the pack until it is time to take them. If you take the tablets out of the pack they may not keep well.
Keep your tablets in a cool dry place where the temperature stays below 25°C, protect from light and moisture. Store in original container.
Do not store VEXAZONE or any other medicine in the bathroom or near a sink. Do not leave it on a windowsill or in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
VEXAZONE tablets come in 3 strengths:
- VEXAZONE 15 mg - white to off-white, round, bi-convex, uncoated tablet debossed with "PG" over 15 on one side and "G" on the other side
- VEXAZONE 30 mg - white to off-white, round, bi-convex, uncoated tablet debossed with "PG" over 30 on one side and "G" on the other side
- VEXAZONE 45 mg - white to off-white, round, bi-convex, uncoated tablet debossed with "PG" over 45 on one side and "G" on the other side.
Each blister pack contains 28 tablets.
Ingredients
VEXAZONE contains 15mg, 30mg or 45mg of pioglitazone hydrochloride as the active ingredient.
The tablets also contain the following inactive ingredients:
- lactose monohydrate
- hypromellose
- colloidal anhydrous silica
- croscarmellose sodium
- polysorbate 80
- magnesium stearate.
VEXAZONE contains sugars as lactose.
Supplier
VEXAZONE is supplied by:
Alphapharm Pty Ltd trading as Viatris
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.viatris.com.au
Phone: 1800 274 276
This leaflet was prepared in May 2024
Australian registration numbers:
VEXAZONE 15mg AUST R 164345
VEXAZONE 30mg AUST R 164344
VEXAZONE 45mg AUST R 164343
VEXAZONE_cmi\May24/02
Published by MIMS July 2024


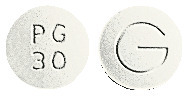




 In the PROactive study, which involved a high risk population of patients with pre-existing macrovascular disease, treatment emergent adverse events that occurred more often in the pioglitazone group compared to placebo group were oedema (26.4% and 15.1%, respectively), hypoglycaemia (27.2% and 18.8%, respectively) and cardiac failure, including serious and nonserious cases (12.6% and 8.7%, respectively).
In the PROactive study, which involved a high risk population of patients with pre-existing macrovascular disease, treatment emergent adverse events that occurred more often in the pioglitazone group compared to placebo group were oedema (26.4% and 15.1%, respectively), hypoglycaemia (27.2% and 18.8%, respectively) and cardiac failure, including serious and nonserious cases (12.6% and 8.7%, respectively). The study population included patients not previously treated with antidiabetic medication (naive; 31%) and patients who were receiving antidiabetic medication at the time of study enrolment (previously treated; 69%). The data for the naive and previously treated patient subsets are shown in Table 5. This run in period was associated with little change in HbA1c and FBG values from screening to baseline for the naive patients. However, for the previously treated group, washout from previous antidiabetic medication resulted in deterioration of glycaemic control and increases in HbA1c and FBG. With pioglitazone, while most patients in the previously treated group had a decrease from baseline in HbA1c and FBG with pioglitazone, in many cases the values did not return to screening levels by the end of the study. The study design did not permit the evaluation of patients who switched directly to pioglitazone from another antidiabetic agent.
The study population included patients not previously treated with antidiabetic medication (naive; 31%) and patients who were receiving antidiabetic medication at the time of study enrolment (previously treated; 69%). The data for the naive and previously treated patient subsets are shown in Table 5. This run in period was associated with little change in HbA1c and FBG values from screening to baseline for the naive patients. However, for the previously treated group, washout from previous antidiabetic medication resulted in deterioration of glycaemic control and increases in HbA1c and FBG. With pioglitazone, while most patients in the previously treated group had a decrease from baseline in HbA1c and FBG with pioglitazone, in many cases the values did not return to screening levels by the end of the study. The study design did not permit the evaluation of patients who switched directly to pioglitazone from another antidiabetic agent. Pioglitazone has been shown to reduce total plasma triglycerides and free fatty acids and to increase HDL cholesterol levels. LDL cholesterol levels remain unchanged. In a 26 week, placebo controlled, dose ranging study, mean triglyceride levels decreased in the pioglitazone 15 mg, 30 mg and 45 mg dose groups compared to a mean increase in the placebo group. Mean HDL levels increased to a greater extent in patients treated with pioglitazone than in the placebo treated patients. There were no consistent differences for LDL and total cholesterol in patients treated with pioglitazone compared to placebo (see Table 6).
Pioglitazone has been shown to reduce total plasma triglycerides and free fatty acids and to increase HDL cholesterol levels. LDL cholesterol levels remain unchanged. In a 26 week, placebo controlled, dose ranging study, mean triglyceride levels decreased in the pioglitazone 15 mg, 30 mg and 45 mg dose groups compared to a mean increase in the placebo group. Mean HDL levels increased to a greater extent in patients treated with pioglitazone than in the placebo treated patients. There were no consistent differences for LDL and total cholesterol in patients treated with pioglitazone compared to placebo (see Table 6). In a separate 24 week study, 260 patients with type 2 diabetes were randomised to one of two forced titration pioglitazone treatment arms (final doses 30 or 45 mg), or a mock titration placebo arm. In one pioglitazone treatment group, patients received an initial dose of 7.5 mg once daily. After four weeks, the dose was increased to 15 mg once daily and after another four weeks, the dose was increased to 30 mg once daily for the remainder of the study (16 weeks). In the second pioglitazone treatment group, patients received an initial dose of 15 mg once daily and were titrated to 30 mg once daily and 45 mg once daily in a similar manner. Treatment with pioglitazone, as described, produced statistically significant improvements in HbA1c and FBG at endpoint compared to placebo (see Table 7).
In a separate 24 week study, 260 patients with type 2 diabetes were randomised to one of two forced titration pioglitazone treatment arms (final doses 30 or 45 mg), or a mock titration placebo arm. In one pioglitazone treatment group, patients received an initial dose of 7.5 mg once daily. After four weeks, the dose was increased to 15 mg once daily and after another four weeks, the dose was increased to 30 mg once daily for the remainder of the study (16 weeks). In the second pioglitazone treatment group, patients received an initial dose of 15 mg once daily and were titrated to 30 mg once daily and 45 mg once daily in a similar manner. Treatment with pioglitazone, as described, produced statistically significant improvements in HbA1c and FBG at endpoint compared to placebo (see Table 7). For patients who had not been previously treated with antidiabetic medication (24%), mean values at screening were 10.1% for HbA1c and 13.22 mmol/L for FBG. At baseline, mean HbA1c was 10.2% and mean FBG was 13.5 mmol/L. Compared with placebo, treatment with pioglitazone titrated to a final dose of 30 mg and 45 mg resulted in reductions from baseline in mean HbA1c of 2.3% and 2.6% and mean FBG of 3.5 mmol/L and 5.28 mmol/L, respectively. For patients who had been previously treated with antidiabetic medication (76%), this medication was discontinued at screening. Mean values at screening were 9.4% for HbA1c and 12 mmol/L for FBG. At baseline, mean HbA1c was 10.7% and mean FBG was 16.11 mmol/L. Compared with placebo, treatment with pioglitazone titrated to a final dose of 30 mg and 45 mg resulted in reductions from baseline in mean HbA1c of 1.3% and 1.4% and mean FBG of 3.06 mmol/L and 3.33 mmol/L, respectively. For many previously treated patients, HbA1c and FBG had not returned to screening levels by the end of the study.
For patients who had not been previously treated with antidiabetic medication (24%), mean values at screening were 10.1% for HbA1c and 13.22 mmol/L for FBG. At baseline, mean HbA1c was 10.2% and mean FBG was 13.5 mmol/L. Compared with placebo, treatment with pioglitazone titrated to a final dose of 30 mg and 45 mg resulted in reductions from baseline in mean HbA1c of 2.3% and 2.6% and mean FBG of 3.5 mmol/L and 5.28 mmol/L, respectively. For patients who had been previously treated with antidiabetic medication (76%), this medication was discontinued at screening. Mean values at screening were 9.4% for HbA1c and 12 mmol/L for FBG. At baseline, mean HbA1c was 10.7% and mean FBG was 16.11 mmol/L. Compared with placebo, treatment with pioglitazone titrated to a final dose of 30 mg and 45 mg resulted in reductions from baseline in mean HbA1c of 1.3% and 1.4% and mean FBG of 3.06 mmol/L and 3.33 mmol/L, respectively. For many previously treated patients, HbA1c and FBG had not returned to screening levels by the end of the study. For patients who had not been previously treated with antidiabetic medication (40%), mean values at screening were 10.3% for HbA1c and 13.33 mmol/L for FBG. At baseline, mean HbA1c was 10.4% and mean FBG was 14.11 mmol/L. Compared with placebo, treatment with pioglitazone 30 mg resulted in reductions from baseline in mean HbA1c of 1.0% and mean FBG of 3.44 mmol/L. For patients who had been previously treated with antidiabetic medication (60%), this medication was discontinued at screening. Mean values at screening were 9.4% for HbA1c and 12 mmol/L for FBG. At baseline, mean HbA1c was 10.6% and mean FBG was 15.94 mmol/L. Compared with placebo, treatment with pioglitazone 30 mg resulted in reductions from baseline in mean HbA1c of 1.3% and mean FBG of 2.56 mmol/L. For many previously treated patients, HbA1c and FBG had not returned to screening levels by the end of the study.
For patients who had not been previously treated with antidiabetic medication (40%), mean values at screening were 10.3% for HbA1c and 13.33 mmol/L for FBG. At baseline, mean HbA1c was 10.4% and mean FBG was 14.11 mmol/L. Compared with placebo, treatment with pioglitazone 30 mg resulted in reductions from baseline in mean HbA1c of 1.0% and mean FBG of 3.44 mmol/L. For patients who had been previously treated with antidiabetic medication (60%), this medication was discontinued at screening. Mean values at screening were 9.4% for HbA1c and 12 mmol/L for FBG. At baseline, mean HbA1c was 10.6% and mean FBG was 15.94 mmol/L. Compared with placebo, treatment with pioglitazone 30 mg resulted in reductions from baseline in mean HbA1c of 1.3% and mean FBG of 2.56 mmol/L. For many previously treated patients, HbA1c and FBG had not returned to screening levels by the end of the study.

