What is in this leaflet
This leaflet answers some common questions about LORSTAT.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have benefits and risks. Your doctor has weighed the risks of you taking LORSTAT against the benefits expected for you.
If you have any concerns about taking this medicine, talk to your doctor or pharmacist.
Keep this leaflet with your medicine. You may need to read it again.
What LORSTAT is used for
LORSTAT is used to treat
- lowers high cholesterol levels
- to reduce the risk of having a heart attack or stroke in people who have high blood pressure and coronary heart disease (CHD) or who are at risk of CHD. Examples of risk factors for CHD include diabetes, a history of stroke, or small blood vessel disease.
What is cholesterol
Everyone has cholesterol in their blood. It is a type of blood fat needed by the body for many things, such as building cell walls, making bile acids (which help to digest food) and some hormones. However, too much cholesterol can be a problem.
Cholesterol is present in many foods and is also made in your body by the liver. If your body makes too much cholesterol or you have too much cholesterol in your diet, then your cholesterol level becomes too high.
High cholesterol is more likely to occur with certain diseases or if you have a family history of high cholesterol.
There are different types of cholesterol. LDL, or low-density lipoprotein, is the 'bad' cholesterol that can block your blood vessels. HDL, or high density lipoprotein cholesterol is the 'good' cholesterol that is thought to remove the 'bad' cholesterol from the blood vessels.
When you have high levels of 'bad' cholesterol in your blood, it may 'stick' to the inside of your blood vessels instead of being carried to the parts of the body where it is needed. Over time, this can form hard areas (called plaques) on the walls of your blood vessels, making it more difficult for the blood to flow. Sometimes, the plaque can detach from the vessel wall and float in the bloodstream; it can then reach a smaller vessel and completely block it. This blocking of your blood vessels can lead to several types of blood vessel disease, heart attack, angina and stroke.
There is another type of fat called triglyceride, which is a source of energy. However, high levels of be associated with low level of 'good' cholesterol and may increase your risk of heart disease.
In some patients, LORSTAT is used to treat high cholesterol and high triglycerides together.
In most people, there are no symptoms of abnormal cholesterol or triglyceride levels. Your doctor can measure your levels with a simple blood test.
How LORSTAT works
LORSTAT belongs to a group of medicines known as HMG-CoA reductase inhibitors. It works by reducing the amount of cholesterol made by the liver. LORSTAT 'bad' cholesterol and can raise the 'good' cholesterol. LORSTAT also helps to protect you from a heart attack or stroke.
When you are taking LORSTAT, you also need to follow a low fat diet and other measures, such as exercise and weight control.
LORSTAT may be used alone, or in combination with other medicines, to treat your condition.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
This medicine is not addictive.
This medicine is available only with a doctor's prescription.
This medicine is not expected to affect your ability to drive a car or operate machinery.
There is not enough information to recommend the use of this medicine for children.
Before you take LORSTAT
When you must not take it
Do not take LORSTAT if you have an allergy to:
- any medicine containing atorvastatin calcium
- any of the ingredients listed at the end of this leaflet
Some of the symptoms of an allergic reaction may include
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin
Do not take this medicine if you have active liver disease.
Do not take this medicine if you are pregnant or intend to become pregnant.
If you are a woman of child-bearing age and are taking this medicine, use a proven method of birth control to avoid pregnancy. It may affect your developing baby if you take it during pregnancy.
Do not breast-feed if you are taking this medicine. The active ingredient in LORSTAT passes into breast milk and there is a possibility that your baby may be affected.
Do not give this medicine to a child. Information of safety and effectiveness in children is limited.
Do not take this medicine if you are taking the antibiotic fusidic acid hemihydrate which is used to treat infections.
Do not take this medicine if you are taking the antivirals, glecaprevir/ pibrentasvir for the treatment of Hepatitis C.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Your doctor will ask you to have your liver function tested before you start to take LORSTAT.
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you have or have had any of the following medical conditions:
- liver problems
- kidney problems
- muscle pain, tenderness or weakness from other medicines used to treat high cholesterol or triglycerides
- a type of stroke called a haemorrhagic stroke or a type of stroke called a lacunar stroke
If you have had one of these strokes before, this medicine may increase the risk of you having a haemorrhagic stroke. - breathing problems
Tell your doctor if you drink alcohol regularly.
Tell your doctor if you are pregnant or plan to become pregnant or are breastfeeding. Your doctor can discuss with you the risks and benefits involved.
If you have not told your doctor about any of the above, tell him/ her before you start taking LORSTAT.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from a pharmacy, supermarket or health food shop.
Some medicines and LORSTAT may interfere with each other. These include:
- digoxin, a medicine used to treat some heart problems
- diltiazem, a medicine used to treat angina
- other medicines to treat high cholesterol or triglycerides
- antacids, medicines used to treat reflux or ulcers
- the antibiotics erythromycin, clarithromycin, rifampicin or fusidic acid hemihydrate
- phenytoin, a medicine used to treat epilepsy (seizures)
- oral contraceptives for birth control
- ciclosporin, a medicine used to suppress the immune system
- some medicines used to treat some fungal infections, such as itraconazole and ketoconazole
- protease inhibitors for the treatment of HIV infection and/or Hepatitis C, such as efavirenz, fosamprenavir, ritonavir, boceprevir, telaprevir, tipranavir/ritonavir, elbasvir/grazoprevir and simeprevir
- HCV non structural protein 5A (NS5A)/5B (NS5B) inhibitors such as daclatasvir and ledipasvir
- letermovir
- spironolactone, a medicine used to treat high blood pressure and certain types of swelling
- vitamin B3
- colchicine, a medicine used to treat a disease with painful, swollen joints caused by uric acid crystals.
These medicines may be affected by LORSTAT or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
How to take LORSTAT
Follow all directions given to you by your doctor and pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the box/bottle, ask your doctor or pharmacist for help.
How much to take
Take LORSTAT only when prescribed by your doctor.
Your doctor will tell you how many tablets you need to take each day. This may depend on your condition and whether or not you are taking any other medicines.
The usual dose of LORSTAT is between 10-80 mg taken once a day.
Follow all directions given to you by your doctor and pharmacist carefully. If you do not understand the instructions ask your doctor or pharmacist for help.
How to take it
Swallow the tablets/capsules whole with a full glass of water.
Do not crush or chew the tablets.
When to take it
Take your medicine at about the same time each day. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
It does not matter if you take this medicine before or after food.
How long to take it
Continue taking your medicine for as long as your doctor tells you to.
This medicine helps to control your condition, but does not cure it. It is important to keep taking your medicine even if you feel well.
If you forget to take it
If it is less than 12 hours before your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take it as soon as you remember, and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for the dose you missed. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much LORSTAT. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
While you are taking LORSTAT
Things you must do
Keep all of your doctor's appointments so that your progress can be checked.
Your doctor will ask you to have your liver function tested from time to time while you are taking LORSTAT to make sure the medicine is working and to prevent unwanted side effects.
Your cholesterol and triglyceride levels also need to be checked regularly while you are taking this medicine.
A regular blood test to check an enzyme called creatine kinase (CK) may be needed. CK in your blood can rise after muscle injury which can be caused by medicines used to treat cholesterol or triglycerides, such as LORSTAT.
If you become pregnant while taking this medicine, stop taking it and contact your doctor immediately.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking LORSTAT.
Tell any other doctors, dentists and pharmacists who treat you that you are taking this medicine.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking this medicine. It may affect other medicines used during surgery.
If you are about to have any blood tests, tell your doctor that you are taking this medicine. It may interfere with the results of some tests.
Things you must not do
Do not take LORSTAT to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop taking your medicine or change the dosage without checking with your doctor. If you stop taking it suddenly, your condition may worsen or you may have unwanted side effects.
If possible, your doctor will gradually reduce the amount you take each day before stopping the medicine completely.
Things to be careful of
Be careful driving or operating machinery until you know how LORSTAT affects you. LORSTAT generally does not cause any problems with your ability to drive a car or operate machinery. However, as with many other medicines, LORSTAT may cause dizziness in some people. If you have any of these symptoms, do not drive, operate machinery or do anything else that could be dangerous.
Be careful when drinking alcohol while you are taking this medicine. Drinking large quantities of alcohol may increase your chance of LORSTAT causing liver problems.
If you feel light-headed, dizzy or faint when getting out of bed or standing up, get up slowly. Standing up slowly, especially when you get up from bed or chairs, will help your body get used to the change in position and blood pressure. If this problem continues or gets worse, talk to your doctor.
Be careful when drinking large quantities of grapefruit juice. Grapefruit juice contains one or more constituents that alter the metabolism of some medicines, including LORSTAT. Therefore, drinking very large quantities of grapefruit juice (over 1 litre) each day increases the chance of LORSTAT causing side effects.
Things that would be helpful for your condition
Some self-help measures suggested below may help your condition. Your doctor or pharmacist can give you more information about these measures.
- Weight: While you are taking LORSTAT, you need to follow a diet plan agreed to with your doctor. This may include measures to lose some weight.
- Exercise: Regular exercise can help lower your cholesterol levels. It is important not to overdo it. Before commencing regular exercise, you should consult your doctor who will suggest the most suitable exercise for you. If you experience any discomfort when exercising, see your doctor.
- Alcohol: Excessive alcohol intake can raise your cholesterol levels or affect your liver function, which could increase the chance of you getting unwanted side effects. Your doctor may discuss with you whether you should reduce the amount of alcohol you drink.
- Smoking: Smoking increases the risk of you suffering from heart problems. Your doctor may advise you to stop smoking.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking LORSTAT.
This medicine helps most people with lowering high cholesterol levels and reducing the risk of having a heart attack or stroke in people who have high blood pressure and coronary heart disease (CHD) or who are at risk of CHD, but it may have unwanted side effects in some people.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by the following list of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- muscle and joint pain, muscle weakness, especially in the forearms, thighs, hips, shoulders, neck, and back
- difficulty climbing stairs or standing up from a chair
- difficulty lifting arms over the head
- falling and difficulty getting up from a fall
- constipation, diarrhoea
- stomach or belly pain, nausea (feeling sick)
- heartburn, indigestion or wind
- urine infection
- headache
- stuffy or runny nose
- nose bleeds
- rash
The above list includes the more common side effects of your medicine. They are usually mild and short-lived.
Tell your doctor as soon as possible if you notice any of the following:
- yellowing of the skin and eyes and dark coloured urine
- feeling weak and tired, excessively thirsty and passing more urine
- problems with breathing, including shortness of breath, persistent cough and fever
The above list includes serious side effects that may require medical attention. Serious side effects are rare.
If any of the following happen, tell your doctor immediately or go to Accident and Emergency at your nearest hospital:
- symptoms of allergy such as skin rash, itching, swelling of the face, lips, mouth, throat or neck which may cause difficulty in swallowing or breathing
- chest pain
- unexpected muscle pain, tenderness or weakness not caused by exercise, particularly if you also feel unwell or have a fever
- sudden severe headache, which may be accompanied by nausea, vomiting, loss of sensation, tingling in any part of the body or ringing in the ears
- severe blisters and bleeding of the lips, eyes, mouth, nose or genitals
The above list includes very serious side effects. You may need urgent medical attention or hospitalisation. These side effects are very rare.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Other side effects not listed above may also occur in some people.
Some of these side effects (for example, changes in thyroid function, cholesterol level or blood pressure) can only be found when your doctor does tests from time to time to check your progress.
After taking LORSTAT
Storage
Keep your tablets in the pack/bottle until it is time to take them. If you take the tablets out of the pack/bottle they may not keep well.
Keep your tablets in a cool dry place where the temperature stays below 25°C.
Do not store LORSTAT or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
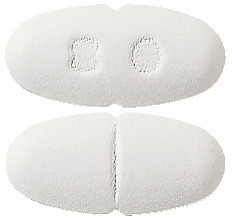
LORSTAT tablets are available in 4 different strengths:
- LORSTAT 10 mg - White, oval, biconvex, film coated tablet plain on one side and debossed '10' on the other side.
- LORSTAT 20 mg - White, oval, biconvex, film coated tablet with break line on one side and debossed '20' on the other side.
- LORSTAT 40 mg - White, oval, biconvex, film coated tablet with break line on one side and debossed '40' on the other side.
- LORSTAT 80 mg - White, oval, biconvex, film coated tablet with break line on one side and debossed '80' on the other side.
Each blister pack contains 30 tablets. Discard blister pack 30 days after the first tablet is taken.
Each bottle pack contains 90 tablets. Discard bottle pack 90 days after opening.
Ingredients
The active ingredient of LORSTAT is atorvastatin (as calcium trihydrate).
It also contains the following inactive ingredients:
- colloidal anhydrous silica
- sodium carbonate
- microcrystalline cellulose
- arginine
- lactose
- croscarmellose sodium
- hyprolose
- magnesium stearate
- Opadry II complete film coating system 85F18378 WHITE (ARTG PI No 12315)
This medicine contains sugars as lactose.
Supplier
LORSTAT is supplied in Australia by:
Alphapharm Pty Ltd trading as Viatris
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
Phone: (02) 9298 3999
www.viatris.com.au
Phone: 1800 274 276
Australian registration numbers:
LORSTAT 10 mg blister pack:
AUST R 334865
LORSTAT 10 mg bottle:
AUST R 334861
LORSTAT 20 mg blister pack:
AUST R 334862
LORSTAT 20 mg bottle:
AUST R 334868
LORSTAT 40 mg blister pack:
AUST R 334863
LORSTAT 40 mg bottle:
AUST R 334867
LORSTAT 80 mg blister pack:
AUST R 334864
LORSTAT 80 mg bottle:
AUST R 334866.
This leaflet was prepared in
December 2023
LORSTAT ® is a Viatris company trade mark
LORSTAT_cmi\Dec23/00
Published by MIMS February 2024




 In three further trials, 1148 patients with either heterozygous familial hypercholesterolaemia, nonfamilial forms of hypercholesterolaemia, or mixed dyslipidaemia were treated with atorvastatin for one year. The results were consistent with those of the dose response study and were maintained for the duration of therapy.
In three further trials, 1148 patients with either heterozygous familial hypercholesterolaemia, nonfamilial forms of hypercholesterolaemia, or mixed dyslipidaemia were treated with atorvastatin for one year. The results were consistent with those of the dose response study and were maintained for the duration of therapy. The primary endpoint examined in ASCOT was the rate of fatal coronary heart disease or nonfatal myocardial infarction over 3.3 years. These coronary events occurred in 1.9% of atorvastatin treated patients compared to 3% of placebo treated patients, a relative risk reduction of 36% (p = 0.0005) (Table 2). Although this difference was statistically significant for the whole trial population, this difference was not statistically significant in specified subgroups such as diabetes, patients with left ventricular hypertrophy (LVH), previous vascular disease or metabolic syndrome.
The primary endpoint examined in ASCOT was the rate of fatal coronary heart disease or nonfatal myocardial infarction over 3.3 years. These coronary events occurred in 1.9% of atorvastatin treated patients compared to 3% of placebo treated patients, a relative risk reduction of 36% (p = 0.0005) (Table 2). Although this difference was statistically significant for the whole trial population, this difference was not statistically significant in specified subgroups such as diabetes, patients with left ventricular hypertrophy (LVH), previous vascular disease or metabolic syndrome.


