SUMMARY CMI
Noumed Bisoprolol
Consumer Medicine Information (CMI) summary
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
1. Why am I using Noumed Bisoprolol?
Noumed Bisoprolol contains the active ingredient bisoprolol fumarate. Noumed Bisoprolol is used to treat heart failure. It is usually used in combination with other medicines. For more information, see Section 1. Why am I using Noumed Bisoprolol? in the full CMI.
2. What should I know before I use Noumed Bisoprolol?
Do not use if you have ever had an allergic reaction to bisoprolol fumarate or any of the ingredients listed at the end of the CMI. Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding. For more information, see Section 2. What should I know before I use Noumed Bisoprolol? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with Noumed Bisoprolol and affect how it works. A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
4. How do I use Noumed Bisoprolol?
- The usual starting dose is 1.25 mg once daily for one week. If well tolerated, your doctor will gradually increase your dose over the next ten weeks.
- The usual dose for maintenance therapy is 10 mg once daily.
- More instructions can be found in Section 4. How do I use Noumed Bisoprolol? in the full CMI.
5. What should I know while using Noumed Bisoprolol?
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Drinking alcohol |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using Noumed Bisoprolol? in the full CMI.
6. Are there any side effects?
Common side effects: tiredness or exhaustion, dizziness, headache, sleep disturbances, nightmares, nausea or vomiting, diarrhoea or constipation, coldness or numbness in the hands or feet.
Serious side effects: muscular weakness or cramps, dizziness or lightheadedness (sometimes with fainting), especially on standing up, which may be due to low blood pressure, a very slow heart beat, hallucinations, depression, skin reactions such as rash, itching, worsening of psoriasis, difficulty hearing, swelling of the face, lips, mouth or throat which may cause difficulty in swallowing or breathing, signs of worsening heart failure such as shortness of breath, sometimes with tiredness or weakness, swelling of the feet or legs due to fluid build up, chest tightness, wheezing, rattly breathing, yellowing of the skin or eyes, dark coloured urine, itching, generally feeling unwell, constant flu‐like symptoms such as fever, sore throat, lack of energy, irregular heart beating. For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
FULL CMI
Noumed Bisoprolol
Active ingredient(s): bisoprolol fumarate
Consumer Medicine Information (CMI)
This leaflet provides important information about using Noumed Bisoprolol. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using Noumed Bisoprolol.
Where to find information in this leaflet:
1. Why am I taking Noumed Bisoprolol?
2. What should I know before I take Noumed Bisoprolol?
3. What if I am taking other medicines?
4. How do I take Noumed Bisoprolol?
5. What should I know while taking Noumed Bisoprolol?
6. Are there any side effects?
7. Product details
1. Why am I taking Noumed Bisoprolol?
Noumed Bisoprolol contains the active ingredient bisoprolol fumarate. Noumed Bisoprolol is a beta‐blocker. It works by affecting the body's response to some nerve impulses, especially in the heart. As a result, it decreases the heart's need for blood and oxygen and therefore reduces the amount of work the heart has to do. NOUMED BISOPROLOL also slows your heart rate, which in turn increases the efficiency of your heart.
Noumed Bisoprolol is used to treat heart failure. It is usually used in combination with other medicines. NOUMED BISOPROLOL can help to reduce the number of heart failure episodes needing hospital admission and also the risk of sudden death.
Heart failure occurs when the heart muscle is weak and unable to pump enough blood to supply the body's needs. Heart failure may start off with no symptoms, but as the condition progresses patients may feel short of breath and notice swelling of the feet and ankles due to fluid build up.
2. What should I know before I take Noumed Bisoprolol?
Warnings
Do not take Noumed Bisoprolol if:
- you are allergic to bisoprolol fumarate, or any of the ingredients listed at the end of this leaflet.
- Always check the ingredients to make sure you can use this medicine.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin - you have or have had any of the following medical conditions
- cardiogenic shock, a serious heart condition causing low blood pressure
- heart conditions where the electrical activity controlling your heart rate does not work properly, causing a very slow heart rate or uneven heart beating
- hypotension, low blood pressure
- severe asthma or chronic obstructive lung disease
- late stages of peripheral arterial occlusive disease
- Raynaud's syndrome, a condition causing numbness, tingling and color change in fingers and toes when exposed to the cold
- untreated phaeochromocytoma, a rare tumour of the adrenal gland
- metabolic acidosis, when there is too much acid in the blood. - It has passed the expiry date printed on pack, or if the packaging is torn or shows signs of tampering.
Check with your doctor if you:
- have any other medical conditions such as:
- asthma, difficulty breathing or other lung problems
- Prinzmetal angina or variant angina
- diabetes
- any allergic conditions
- psoriasis, a skin disease with thickened patches of red skin, often with silvery scales
- hyperthyroidism, an over active thyroid gland
- any blood vessel disorder causing poor circulation in the arms and legs
- kidney problems
- liver problems
- phaeochromocytoma, a rare tumour of the adrenal gland. - take any medicines for any other condition
- plan to have surgery
The anaesthetist must be told that you are taking NOUMED BISOPROLOL before surgery, in order to allow for your condition and medications.
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant.
Talk to your doctor if you are breastfeeding or intend to breastfeed. NOUMED BISOPROLOL is not recommended while you are breastfeeding.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interfere with Noumed Bisoprolol and affect how it works.
- calcium antagonists, medicines used to treat high blood pressure and angina such as diltiazem, verapamil
- clonidine, a medicine used to treat high blood pressure
- monoamine oxidase inhibitors, medicines used to treat depression such as phenelzine, tranylcypromine
- anti‐arrhythmic drugs used to treat irregular or abnormal heartbeat such as flecainide, amiodarone, disopyramide
- certain medicines used to treat arthritis, pain or inflammation such as indomethacin or ibuprofen
- other beta‐blockers, including eye drops
- insulin and oral drugs for diabetes
- anaesthetic agents used in surgery
- digoxin, a medicine used to treat heart failure
- ergot derivatives, medicines commonly used to treat migraines
- tricyclic antidepressants
- barbiturates, medicines used to treat epilepsy
- phenothiazines, a type of medicine used to treat some mental conditions
- rifampicin, a medicine use to treat tuberculosis
- mefloquine, a medicine used to treat malaria
- adrenaline, a medicine used to treat allergic reactions.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect Noumed Bisoprolol.
4. How do I take Noumed Bisoprolol?
How much to take
- The usual starting dose is 1.25 mg once daily for one week. If well tolerated, your doctor will gradually increase your dose over the next ten weeks. The usual dose for maintenance therapy is 10 mg once daily.
- Follow the instructions provided and use Noumed Bisoprolol until your doctor tells you to stop.
- Ask your doctor or pharmacist if you are unsure of the correct dose for you.
- They will tell you exactly how much to take.
- Follow the instructions they give you.
- If you take the wrong dose, NOUMED BISOPROLOL may not work as well and your problem may not improve.
When to take Noumed Bisoprolol
- Take your medicine during or immediately after food in the morning. This will lessen the chance of side effects.
How to take Noumed Bisoprolol
Swallow the tablets with a glass of water. Do not crush or chew the tablets.
If you need to break NOUMED BISOPROLOL, hold tablet with both hands and snap along break line.
Continue taking your medicine for as long as your doctor tells you.
This medicine helps to control your condition, but does not cure it. It is important to keep taking your medicine every day, usually as long term treatment. It is very important that you do not stop taking NOUMED BISOPROLOL suddenly.
If you forget to take Noumed Bisoprolol
Noumed Bisoprolol should be taken regularly at the same time each day. If you miss your dose at the usual time, take your dose as soon as you remember, and continue to take it as you would normally.
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Do not take a double dose to make up for the dose you missed. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much Noumed Bisoprolol
If you think that you have taken too much Noumed Bisoprolol, you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre
(by calling 13 11 26), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
Symptoms of an overdose may include dizziness, very slow heart rate, difficulty breathing and shock.
5. What should I know while taking Noumed Bisoprolol?
Things you should do
If you become pregnant while taking this medicine, tell your doctor immediately.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking NOUMED BISOPROLOL.
Tell any other doctors, dentists, and pharmacists who treat you that you are taking this medicine.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking this medicine. It may affect other medicines used during surgery.
If you are about to have any blood tests, tell your doctor that you are taking this medicine.
It may interfere with the results of some tests.
Keep all of your doctor's appointments so that your progress can be checked.
Your doctor may check your eyes, thyroid, lipid and blood glucose levels from time to time to make sure the medicine is working and to prevent unwanted side effects.
Tell your doctor if, for any reason, you have not taken your medicine exactly as prescribed.
Your doctor may think it is not working effectively and change your treatment unnecessarily.
Remind any doctor, dentist or pharmacist you visit that you are using Noumed Bisoprolol.
If you feel light‐headed, dizzy or faint when getting out of bed or standing up, get up slowly.
Standing up slowly, especially when you get up from bed or chairs, will help your body get used to the change in position and blood pressure. If this problem continues or gets worse, talk to your doctor.
Things you should not do
- Do not stop using this medicine suddenly.
- Do not take NOUMED BISOPROLOL to treat any other complaints unless your doctor tells you to.
- Do not give your medicine to anyone else, even if they have the same condition as you.
- Do not stop taking your medicine or lower the dosage without checking with your doctor.
- Stopping NOUMED BISOPROLOL suddenly may cause your condition to worsen or other heart complications may occur. NOUMED BISOPROLOL should only be reduced gradually over a period of about two weeks before stopping completely.
Diabetes
- If you are being treated for diabetes, make sure you check your blood sugar level regularly and report any changes to your doctor.
- NOUMED BISOPROLOL may change how well your diabetes is controlled. It may also cover up some of the symptoms of low blood sugar, called hypoglycaemia, such as fast heart beat. NOUMED BISOPROLOL may make hypoglycaemia last longer. Your dose of diabetic medicines, including insulin, may need to change.
- Take this medicine with caution under strict fasting conditions.
- Ask your doctor or pharmacist to answer any questions you may have.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how Noumed Bisoprolol affects you.
Noumed Bisoprolol may cause dizziness in some people, especially after the first dose. If you have any of these symptoms, do not drive, operate machinery or do anything else that could be dangerous.
Drinking alcohol
Tell your doctor if you drink alcohol.
If you drink alcohol, dizziness or light‐headedness may be worse.
Looking after your medicine
- Keep your medicine in the original container. If you take it out of its original container it may not keep well.
- Keep your medicine in a cool dry place where the temperature stays below 25°C.
Follow the instructions in the carton on how to take care of your medicine properly.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on window sills.
Keep it where young children cannot reach it.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
| Speak to your doctor if you have any of these less serious side effects and they worry you. |
Serious side effects
| Serious side effects | What to do |
| Call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What Noumed Bisoprolol contains
| Active ingredient (main ingredient) | Bisoprolol fumarate |
| Other ingredients (inactive ingredients) | calcium hydrogen phosphate microcrystalline cellulose pregelatinised maize starch croscarmellose sodium colloidal anhydrous silica magnesium stearate Opadry II OY‐L‐28900 iron oxide yellow (5 & 10 mg tablets only) iron oxide red (10 mg tablet only) |
| Potential allergens | lactose |
Do not take this medicine if you are allergic to any of these ingredients.
What Noumed Bisoprolol looks like
NOUMED BISOPROLOL 2.5mg Film Coated Tablets: White coloured, round, snap‐tab film‐coated tablet, divisible in two parts, with a one‐sided embossment “BIS 2.5”. Each film‐coated tablet contains 2.5 mg bisoprolol fumarate.
(AUST R 285578)
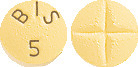
NOUMED BISOPROLOL 5mg Film Coated Tablets: Yellow coloured, round, snap‐tab film‐coated tablet, divisible in four parts, with a one‐sided embossment “BIS 5”. Each film‐coated tablet contains 5 mg bisoprolol fumarate.
(AUST R 285579)
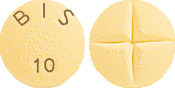
NOUMED BISOPROLOL 10mg Film Coated Tablets: Apricot coloured, round, snap‐tab film‐coated tablet, divisible in four parts, with a one‐sided embossment “BIS 10”. Each film‐coated tablet contains 10 mg bisoprolol fumarate.
(AUST R 285580)
NOUMED BISOPROLOL tablets are available in blisters
(Al/Al) of 28 tablets.
Who distributes Noumed Bisoprolol
Sponsor
Avallon Pharmaceuticals Pty Ltd
Level 5, 7 Eden Park Drive
Macquarie Park NSW 2113
Tel: 1800 930 999
www.avallon-pharma.com.au
This leaflet was prepared in August 2022.
Published by MIMS October 2022



 Chemical name: (2RS)‐1‐[4‐[[2‐(1‐Methylethoxy)ethoxy]methyl]phenoxy]‐3‐[(1methylethyl)amino]propan‐2‐ol fumarate.
Chemical name: (2RS)‐1‐[4‐[[2‐(1‐Methylethoxy)ethoxy]methyl]phenoxy]‐3‐[(1methylethyl)amino]propan‐2‐ol fumarate.