WHAT IS IN THIS LEAFLET
This leaflet answers some common questions about Adempas. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking Adempas against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
WHAT ADEMPAS IS USED FOR
Adempas is used to treat adults with high blood pressure in the lung vessels caused by:
- blood clots in the lungs (known as chronic thromboembolic pulmonary hypertension or CTEPH)
- narrowing of the vessels that carry blood from the heart to the lungs (known as pulmonary arterial hypertension or PAH)
If the high blood pressure in the lung vessels is caused by CTEPH it can sometimes be treated with surgery. Adempas may be used if surgery is not possible or if there is still high blood pressure in the lung vessels after surgery.
High blood pressure in the lung vessels means that the heart needs to work harder to pump blood through the lungs. This may cause you to feel tired, dizzy and short of breath.
Adempas contains the active substance riociguat. Riociguat is a soluble guanylate cyclase (sGC)-stimulator.
Adempas relaxes the lung vessels making it easier for the heart to pump blood through them. This lowers the pressure in these vessels, relieves the symptoms, and can lead to an improvement in the ability to exercise and perform physical tasks.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
This medicine is only available with a doctor’s prescription.
BEFORE YOU TAKE ADEMPAS
When you must not take it
Do not take Adempas if you have an allergy to:
- riociguat, the active ingredient in Adempas
- any of the ingredients listed at the end of this leaflet
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin
Do not take this medicine if you are pregnant, think you might be pregnant or if you could become pregnant because you are not using reliable birth control (contraception). It may affect the development of your baby if you take it during pregnancy or if you become pregnant within one month of stopping Adempas.
If you could become pregnant, your doctor will ask you to take a pregnancy test before you start Adempas.
Women must not become pregnant for at least 1 month after stopping treatment with Adempas. Do not use Adempas if you are breast-feeding. If you are breast-feeding, ask your doctor or pharmacist for advice before taking Adempas because it might harm your baby. A decision must be made whether to discontinue breast feeding or to stop therapy with Adempas.
Do not take this medicine if you are taking nitrate medicines which include:
- glyceryl trinitrate (also called nitroglycerine)
- nicorandil
- sodium nitroprusside
- isosorbide mononitrate
- isosorbide dinitrate
- amyl nitrite (also known as ‘poppers’, ‘amyl’ or ‘rush’)
Do not take this medicine if you are taking:
- medicines known as PDE-5 inhibitors, such as sildenafil, vardenafil or tadalafil (used to treat high blood pressure in lung vessels in men and women and/or erectile dysfunction in men)
- medicines known as non-specific PDE inhibitors, such as dipyridamole or theophylline used in cardiovascular and respiratory conditions
- other soluble guanylate cyclase stimulators. Ask your doctor if you are not sure if you are taking a soluble guanylate cyclase stimulator
Do not take this medicine if you have increased pressure in your pulmonary circulation associated with scarring of the lungs, of unknown cause (idiopathic pulmonary pneumonia).
Do not take this medicine after the expiry date printed on the pack and blister. The expiry date is printed on the carton and on each blister after “EXP” (e.g. 11 18 refers to November 2018). The expiry date refers to the last day of that month. If it has expired return it to your pharmacist for disposal.
Do not take this medicine if the packaging is torn or shows signs of tampering. If the packaging is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Adempas is not recommended for children or adolescents. The safety and efficacy of Adempas has not been proven in patients less than 18 years of age.
Before you start to take it
Tell your doctor if you have allergies to any medicines, foods, preservatives or dyes.
Tell your doctor if you have or have had any of the following medical conditions:
- recent serious bleeding, especially from the lungs, or if you have undergone treatment to stop coughing up blood (bronchial arterial embolization)
- problems with your heart and circulation
- low blood pressure
- liver disease
- kidney disease
Tell your doctor if you are pregnant, or think you might be pregnant or if you could become pregnant because you are not using reliable birth control (contraception).
Women who may become pregnant should have a negative pregnancy test prior to starting treatment with Adempas. If there is a chance that you could become pregnant, use at least two reliable forms of birth control (contraception) while you are taking Adempas. You must continue to use contraception for at least 1 month after stopping Adempas. Speak with a gynaecologist for contraceptive advice if you are unsure of what to do.
Tell your doctor if you are breast-feeding or planning to breast-feed. Adempas should not be used while breast-feeding. If you are breast-feeding, ask your doctor or pharmacist for advice before taking Adempas because it might harm your baby. A decision must be made whether to discontinue breast feeding or to stop therapy with Adempas.
Tell your doctor if you smoke. The metabolism of Adempas is affected by smoking, making it less effective. You should not smoke while taking Adempas.
If you have not told your doctor about any of the above, tell him/her before you start taking Adempas.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Do not take this medicine if you are taking:
- medicines known as nitrate medicine such as glyceryl trinitrate, isosorbide mononitrate, nicorandil, amyl nitrite (also known as ‘poppers’, ‘amyl’ or ‘rush’) or sodium nitroprusside
- medicines known as PDE-5 inhibitors, such as sildenafil, vardenafil or tadalafil (used to treat high blood pressure in lung vessels in men and women and/or erectile dysfunction in men)
- medicines known as non-specific PDE inhibitors, such as dipyridamole or theophylline used in cardiovascular and respiratory conditions
- soluble guanylate cyclase stimulators
In particular, tell your doctor if you are taking:
- medicines known as protease inhibitors for the treatment of HIV infection, such as rilpivirine, abacavir, efavirenz and ritonavir
- medicines used to treat fungal infections such as ketoconazole or itraconazole
Also tell your doctor if you are taking:
- cancer medicines called tyrosine kinase inhibitors such as erlotinib or gefitinib
- cyclosporin, a medicine used to prevent rejection of transplanted organs
- epoprostenol, a medicine used to treat pulmonary arterial hypertension
- antacids, used to treat heartburn (indigestion)
- granisetron, a medicine used to prevent nausea and vomiting
- medicines used to treat epilepsy, e.g. phenytoin, carbamazepine, phenobarbitone
- anticoagulants, which are medicines used to prevent blood clots
- other medicines used to treat blood pressure
- clarithromycin, an antibiotic
- St. John’s Wort, a herbal treatment for depression
These medicines may be affected by Adempas or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking Adempas.
HOW TO TAKE ADEMPAS
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information in this leaflet.
If you do not understand the instructions printed on the label, ask your doctor or pharmacist for help.
How much to take
The recommended starting dose of Adempas is one 1 mg tablet, three times daily for 2 weeks.
Your doctor may advise you to start on a lower dose. Take Adempas exactly as your doctor tells you.
After 2 weeks, your doctor will adjust your dose depending on your blood pressure and your response to the medicine. At the start of treatment your doctor will measure your blood pressure at least every two weeks.
If you have no side effects and your blood pressure is above a certain level, your doctor will increase your dose every 2 weeks. The doctor may increase your dose up to a maximum of 2.5 mg three times a day.
Tell your doctor if you have any side effects as they may need to lower your dose.
How to take it
Swallow the tablet whole with water.
Adempas can be taken with or without food.
When to take it
Take your medicine three times a day at about the same time each day. Taking it at the same time each day will have the best effect. It will also help you remember when to take it. Each dose should be 6 to 8 hours apart. It does not matter if you take this medicine before or after food.
If you are taking an antacid (a medicine to treat stomach disease or heartburn), take it at least 1 hour after taking Adempas.
Transitioning between sildenafil or tadalafil and Adempas
Stop taking sildenafil at least 24 hours before you start taking Adempas.
Stop taking tadalafil at least 48 hours before you start taking Adempas.
Stop taking Adempas at least 24 hours before you start taking sildenafil or tadalafil.
How long to take it
Continue taking Adempas for as long as your doctor tells you. Adempas helps to control your condition, but does not cure it. It is important to keep taking Adempas even if you feel well.
In case treatment has to be interrupted for 3 days or more, please contact your doctor before restarting Adempas.
If you forget to take it
If it is almost time for your next dose, skip the dose you missed and take the next dose when you are meant to.
Do not take a double dose to make up for the dose that you missed. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (Australia: 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much Adempas. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
WHILE YOU ARE TAKING ADEMPAS
Things you must do
If you are about to start on any new medicine, remind your doctor and pharmacist that you are taking Adempas.
Tell any other doctors, dentists, and pharmacists who treat you that you are taking this medicine.
Tell your doctor if your shortness of breath is getting worse during treatment with Adempas. This can be caused by a build-up of fluid in the lungs due to another lung vessel disease, known as pulmonary veno-occlusive disease.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking this medicine. It may affect other medicines used during surgery.
If there is a chance you could become pregnant, you should have a negative pregnancy test monthly during treatment and one month after you stop taking Adempas. You should use two reliable forms of contraception while you are taking Adempas. If you become pregnant while taking Adempas, tell your doctor immediately. You must continue to use contraception for at least 1 month after stopping Adempas.
Do not smoke while taking Adempas. Tell your doctor if you stop or start smoking during treatment. Smoking may reduce the effectiveness of Adempas and your doctor may have to adjust your dose if required.
If treatment is interrupted for 3 days or more, contact your doctor before restarting Adempas.
Keep all of your doctor’s appointments so that your progress can be checked. Your doctor will measure your blood pressure and do other tests from time to time to make sure the medicine is working and to check for unwanted side effects.
Things you must not do
Do not take Adempas to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop taking your medicine or lower the dose without checking with your doctor. If you stop taking it, your condition may worsen.
Things to be careful of
Be careful driving or operating machinery until you know how Adempas affects you. Dizziness is a common side effect of Adempas. If you experience dizziness during treatment, do not drive or operate machinery.
If you feel light-headed, dizzy or faint when getting out of bed or standing up, get up slowly. Standing up slowly, especially when you get up from bed or chairs, will help your body get used to the change in position and blood pressure.
Tell your doctor if you feel dizzy or faint. They may want to check your blood pressure.
SIDE EFFECTS
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Adempas.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- light headedness (hypotension)
- dizziness
- headache
- indigestion
- diarrhoea
- nausea
- vomiting
- swelling of extremities (hands, ankles or feet)
- tiredness (may be due to decrease in red blood cells)
- anaemia (decrease in red blood cells)
- fast or irregular heart beats
- nosebleed
- nasal congestion
- heartburn
- difficulty in swallowing
- constipation
- stomach pain
- stomach bloating
The above list includes the more common side effects of your medicine.
If any of the following happen, tell your doctor immediately or go to Accident and Emergency at your nearest hospital:
- coughing up blood
The most serious side effects are coughing up blood and bleeding from the lungs which can be fatal.
You may need urgent medical attention or hospitalisation. These side effects are not common.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Other side effects not listed above may also occur in some people.
AFTER TAKING ADEMPAS
Storage
Keep your tablets in the pack until it is time to take them. If you take the tablets out of the pack they may not keep well.
Keep the medicine in a cool dry place where the temperature stays below 30°C.
Do not store it or any other medicine in the bathroom, near a sink, or on a window-sill.
Do not leave it in the car. Heat and damp can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking Adempas or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
PRODUCT DESCRIPTION
What it looks like
Adempas tablets are round film-coated tablets supplied in blister packs of 21, 42 or 84 tablets (not all pack sizes may be marketed).
Adempas 0.5 mg tablets: White, tablets marked with the Bayer cross on one side and 0.5 and an “R” on the other side.
Adempas 1 mg tablets: Pale yellow, tablets marked with the Bayer cross on one side and 1 and an “R” on the other side.
Adempas 1.5 mg tablets: Yellow-orange, tablets marked with the Bayer cross on one side and 1.5 and an “R” on the other side.
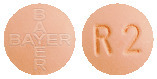
Adempas 2 mg tablets: Pale orange, tablets marked with the Bayer cross on one side and 2 and an “R” on the other side.
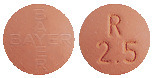
Adempas 2.5 mg tablets: Red-orange, tablets marked with the Bayer cross on one side and 2.5 and an “R” on the other side.
Ingredients
Active ingredients:
- Adempas 0.5 mg contains 0.5 mg of riociguat
- Adempas 1 mg contains 1 mg of riociguat
- Adempas 1.5 mg contains 1.5 mg of riociguat
- Adempas 2 mg contains 2 mg of riociguat
- Adempas 2.5 mg contains 2.5 mg of riociguat
Inactive ingredients:
- cellulose microcrystalline
- crospovidone
- hypromellose
- lactose monohydrate
- magnesium stearate
- sodium lauryl sulfate
- hyprolose
- propylene glycol
- titanium dioxide
- iron oxide yellow (1 mg, 1.5 mg, 2 mg and 2.5 mg)
- iron oxide red (2 mg and 2.5 mg)
Suppliers
Made in Germany for:
Bayer Australia Ltd
ABN 22 000 138 714
875 Pacific Highway
Pymble NSW 2073
www.bayer.com.au
Australian registration number
Adempas 0.5 mg
- AUST R 207595
Adempas 1 mg
- AUST R 207599
Adempas 1.5 mg
- AUST R 207597
Adempas 2 mg
- AUST R 207598
Adempas 2.5 mg
- AUST R 207596
Date of preparation
June 2022
See TGA website (www.ebs.tga.gov.au) for latest Australian Consumer Medicine Information.
® Registered Trademark of the Bayer Group, Germany
© Bayer Australia Ltd
All rights reserved.

Published by MIMS July 2022







 Last visit = last observed value, not including follow-up, for patients who completed the study or withdrew, except imputed worst value (zero) in case of death or clinical worsening without a termination visit or a measurement at that termination visit.
Last visit = last observed value, not including follow-up, for patients who completed the study or withdrew, except imputed worst value (zero) in case of death or clinical worsening without a termination visit or a measurement at that termination visit. No statistically significant (below threshold of hierarchical testing1) result was found for:
No statistically significant (below threshold of hierarchical testing1) result was found for:

 Last visit = last observed value, not including follow-up, for patients who completed the study or withdrew, except imputed worst value (zero) in case of death or clinical worsening without a termination visit or a measurement at that termination visit.
Last visit = last observed value, not including follow-up, for patients who completed the study or withdrew, except imputed worst value (zero) in case of death or clinical worsening without a termination visit or a measurement at that termination visit.



 C20H19FN8O2.
C20H19FN8O2.