Key points
- On 1 December 2022, vericiguat (Verquvo) for chronic heart failure was added to the PBS General Schedule (Section 85).
It must be initiated by a cardiologist, or a medical practitioner such as a GP who has been directed by a cardiologist. It can be continued by a medical practitioner or nurse practitioner. - Vericiguat is PBS listed for patients with chronic heart failure with LVEF < 45% and NYHA class II–IV, stabilised after a decompensation event requiring either hospitalisation or IV diuretic therapy under certain circumstances.
It must be add-on therapy to optimal standard treatment, which must include, unless contraindicated or not tolerated; an ACE inhibitor or ARB or ARNI, and a beta blocker. - Vericiguat belongs to a novel class of medicines called sGC stimulators.
Strengths available are 2.5 mg, 5 mg and 10 mg tablets. The start dose, 2.5 mg once daily, should be doubled every 2 weeks to the target maintenance dose of 10 mg once daily. It should be taken with food. - Current Australian guidance recommends vericiguat as add-on therapy for persistent HFrEF.
Recommended standard treatment for HFrEF, defined as LVEF ≤ 40%, is an ARNI (or ACE inhibitor), heart failure beta blocker and MRA up-titrated to the maximum tolerated dose, and an SGLT2 inhibitor. - Vericiguat has improved health benefits when added to standard treatment and a tolerable side effect profile.
The key study evidence (VICTORIA trial) for the PBS listing found a 3% absolute risk reduction for the primary outcome – first occurrence of hospitalisation for heart failure or cardiovascular death – which was mostly driven by reduced hospitalisations. Although there was a small increase in drug-related adverse events compared with placebo, vericiguat appeared to be reasonably well tolerated.
Abbreviations
|
ACE - angiotensin-converting enzyme ARB - angiotensin receptor blocker ARNI - angiotensin receptor neprilysin inhibitor ARR - absolute risk reduction cGMP - cyclic guanosine monophosphate eGFR - estimated glomerular filtration rate HFmrEF - heart failure with mildly reduced ejection fraction HFrEF - heart failure with reduced ejection fraction IV - intravenous LVEF - left ventricular ejection fraction MRA - mineralocorticoid receptor antagonist NO - nitric oxide NT-proBNP - N-terminal pro hormone B-type natriuretic peptide NYHA - New York Heart Association PBAC - Pharmaceutical Benefits Advisory Committee PBS - Pharmaceutical Benefits Scheme sGC - soluble guanylate cyclase SGLT2 - sodium–glucose co-transporter-2 TGA - Therapeutic Goods Administration |
Evidence snapshot
What is known about this medicine?
Vericiguat is TGA-approved and PBS-listed as an add-on to optimal standard treatment for people with chronic heart failure with left ventricular ejection fraction (LVEF) < 45% who are stabilised after a recent decompensation event.
In the key study trial of 5050 people with heart failure who had LVEF < 45% and were stabilised after a recent decompensation event, the use of standard treatment medicines was: 93% taking a beta blocker, 73% angiotensin converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB), 70% mineralocorticoid receptor antagonist (MRA), 15% angiotensin receptor neprilysin inhibitor (ARNI) and 3% sodium–glucose co-transporter-2 (SGLT2) inhibitor.
When vericiguat was added to standard treatment, the trial found the relative hazard of the primary outcome (time to first occurrence of hospitalisation for heart failure or cardiovascular death) over a median duration of follow up of 10.8 months was reduced by 10% compared to placebo, with a 3% absolute risk reduction.
What’s new with heart failure?
In the 2018 Australian heart failure guidelines, the definition and classification of heart failure with reduced ejection fraction (HFrEF) was LVEF < 50%, with mildly reduced ejection fraction (LVEF 41–49%; HFmrEF) included for treatment purposes under the HFrEF diagnosis. A 2022 guidance update defines HFrEF as LVEF ≤ 40% and separates HFmrEF for treatment purposes.
The 2018 guidelines’ standard treatment for HFrEF was three medicines: an ACE inhibitor (or ARB), heart failure beta blocker and MRA up-titrated to maximum tolerated doses.
The 2022 guidance update recommends four medicines for standard treatment of HFrEF: an ARNI (or ACE inhibitor), heart failure beta blocker and MRA up-titrated to maximum tolerated dose and a SGLT2 inhibitor. The recommended standard treatment for HFmrEF is similar to HFrEF.
Each of the four medicines should be started as soon as clinically possible and this can include starting two medicines simultaneously. For example, for people with HFrEF who are congested, the recommended first step is to start an ARNI and SGLT2 inhibitor because the evidence shows the benefits occur early with these medicines.
The 2022 guidance update recommends vericiguat as add-on therapy to standard treatment for persistent HFrEF (LVEF that remains ≤ 40% in response to optimal standard treatment and ongoing symptoms/signs and/or limited functional capacity) with a history of recent hospitalisation and high risk of readmission.
Areas of uncertainty
When adding vericiguat for people already receiving standard treatment that includes an ARNI and SGLT2 inhibitor, the degree of benefit is unknown due to limited exposure to these two medicines in the key study trial.
In the sub-group analysis of the key study trial, people with more severe disease and older age (≥ 75 years) had worse primary outcome results compared to placebo. These sub-groups may be more likely to be included in the vericiguat PBS listing patient population than those investigated in the key study trial. However, given prescribing is restricted to under the supervision of a cardiologist the risk of use in these sub-groups should be reduced.
What does NPS MedicineWise say?
The PBS listing for vericiguat is likely to include patients with more severe disease, who may not experience as high a benefit with vericiguat as the key study trial population.
The PBS listings for vericiguat, SGLT2 inhibitors and ARNI for chronic heart failure differ from the 2022 guidance with regards to standard treatment.
Standard treatment for PBS-subsidised medicines requires a more stepwise approach compared to the 2022 guidance update and starts with different medicines.
The PBS listings require optimal standard treatment with a beta blocker and ACE inhibitor (or ARB or ARNI) before starting vericiguat or an SGLT2 inhibitor, and optimal standard treatment with at least an ACE inhibitor (or ARB) before starting an ARNI.
The 2022 guidance update is clear about the importance of starting an ARNI and SGLT2 inhibitor as soon as clinically possible and this can include starting two medicines simultaneously.
PBS listing
On 1 December 2022, vericiguat for chronic heart failure was added to the General Schedule (Section 85).1 See Table 1 for the eligibility criteria.
|
Initial treatment |
Continuing treatment |
|
|
PBS Authority |
Authority Required (telephone/online) |
Authority Required (Streamlined) |
|
Permitted prescribers |
|
|
|
Treatment criteria |
Patient must already be receiving optimal standard chronic heart failure treatment, which must include, unless contraindicated according to the TGA-approved Product Information or cannot be tolerated, a:
|
|
|
Clinical criteria |
Patient must:
Patient must have had a decompensation eventa requiring at least one of:
Patient must be stabilised after decompensation event, which is defined as having none of the following:
|
Patient must have previously received PBS-subsidised treatment with this medicine for this condition. |
a Date of the decompensation event and initiation of treatment with vericiguat must be documented in the patient’s medical records when PBS-subsidised treatment is initiated.
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; ARNI = angiotensin receptor neprilysin inhibitor; IV = intravenous; NYHA = New York Heart Association
Grandfathered treatment
Patients who have received non-PBS-subsidised treatment with vericiguat for chronic heart failure prior to 1 December 2022 may qualify for initial PBS-subsidised treatment (Authority Required) under this restriction (once only) if they:1
- satisfy all of the above clinical and treatment criteria for initial treatment prior to initiating non-PBS-subsidised treatment with vericiguat for chronic heart failure, and
- are treated by a cardiologist or a medical practitioner such as a GP who has been directed to prescribe vericiguat by a cardiologist.
For continuing PBS-subsidised treatment under a grandfathering provision, patients must qualify under the continuing treatment above.1
This grandfathering restriction will cease to operate on 1 December 2023, which is 12 months after the PBS listing date.1
What is it?
Vericiguat belongs to a novel class of medicines called soluble guanylate cyclase (sGC) stimulators.2-4
It is TGA approved and available on the PBS as an add-on to optimal standard treatment for adults with symptomatic chronic heart failure with LVEF < 45% who are stabilised after a recent heart failure decompensation event requiring admission and/or intravenous (IV) diuretic therapy. The strengths available are 2.5 mg, 5 mg and 10 mg tablets.1,2
The only other TGA-registered sGC stimulator is riociguat, which is PBS-listed as a S100 Highly Specialised Drugs medicine for pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension.5,6
Mechanism of action
sGC is an enzyme that interacts with nitric oxide (NO) to produce cyclic guanosine monophosphate (cGMP).7,8
The resultant NO-sGC-cGMP signalling pathway improves diastolic relaxation and reduced hypertrophy, inflammation and fibrosis. Impairment of this pathway, as a result of conditions such as heart failure, increases myocardial and vascular dysfunction.7,8
Vericiguat acts as a direct stimulator of sGC independent of NO. It may be effective in restoring the NO-sGC-cGMP signalling pathway in a state of low NO.7,8 Vericiguat may also enhance sGC sensitivity to endogenous NO to exert more physiological action and produce cGMP in a state of low NO.8
Increasing sGC and restoring the NO-sGC-cGMP signalling pathway can induce relaxation of the blood vessels and improve myocardial and vascular dysfunction.8
Who is it for?
Vericiguat is TGA approved for adults with symptomatic chronic heart failure with LVEF < 45% who are stabilised after a recent worsening as add-on therapy to standard treatment (note that the product information uses the term ‘standard of care therapy’). This indication was registered in November 2021.2
Precautions
Vericiguat should not be prescribed for patients:2
- with severe hepatic impairment or an estimated glomerular filtration rate (eGFR) < 15 mL/min/1.73 m2 or those on dialysis
- < 18 years of age
- with hypersensitivity to the active substance or any of the excipients, including lactose.
Treatment should not be initiated in patients with systolic blood pressure < 100 mmHg.2
Before starting vericiguat, the volume status should be optimised with diuretic therapy to stabilise the patient after the decompensation event.2
Where does it fit?
The most recent Australian guidelines for heart failure, including HFrEF, were published in 2018 by the National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand. Vericiguat was not included in the guidelines because it was not registered at the time in Australia nor overseas.9
Since 2018, several important changes for the definition and standard treatment of HFrEF have been recommended.
These changes include guidance on the place in therapy for vericiguat. There are some differences between the PBS clinical criteria and guidance for vericiguat, particularly with regards to standard treatment.
What’s new since the 2018 Australian guidelines?
Definition of HFrEF and HFmrEF
In the 2018 Australian guidelines HFrEF was defined as LVEF < 50%. LVEF 41–49%, described as HFmrEF, was included for treatment purposes under the HFrEF diagnosis.10
In 2021, three international heart failure specialist societies (from the USA, Europe and Japan) published an update to the classification and definitions for HFrEF. This update was endorsed by the Cardiac Society of Australia and New Zealand.11
In the updated classification:11
- HFrEF is defined as LVEF ≤ 40%
- HFmrEF (LVEF 41–49%) is separate to HFrEF for treatment purposes.
Standard treatment for HFrEF
Since 2018, a number of clinical trials have evaluated several novel medicines for HFrEF. Their results have led to updates of heart failure guidelines in Europe in 2021 and the USA in 2022.12-14
In 2022 an academic group of Australian heart failure clinicians developed a consensus statement update on the management of heart failure. This statement addressed a gap in practice as current guidelines were from 2018 and not scheduled for a review. The statement was published in August 2022 and includes the classification and definitions developed by the 2021 international update.12
The recommendations for the standard treatment for HFrEF in the 2018 Australian guidelines compared to the 2022 consensus statement are described in Table 2.
For both sets of recommendations, there are two pathways for pharmacological management that are differentiated by the patient’s volume status: congested or euvolaemic. These two pathways converge for all patients who have ‘persistent HFrEF’.10,12 See Figure 1.
Table 2: Recommended standard treatment for HFrEF – Australian 2018 guidelines compared to the 2022 consensus statement10,12
|
2018 guidelines |
2022 consensus statement |
||
|
Medicines included (one of each class) |
|
|
|
|
Target |
Each medicine should be up-titrated to target or maximum tolerated dose.b See NPS MedicineWise Up-titrating heart failure medicines: A practical guide for start and target doses of recommended medicines for heart failure beta blockers, ARNIs, ACE inhibitors and MRAs. |
||
|
See NPS MedicineWise RADAR articles about dapagliflozin for HFrEF and empagliflozin for HFrEF. These SGLT2 inhibitors are single dose and hence, do not need up-titration. |
|||
|
Start and up-titration guidance |
Start at low doses, double the dose one medicine at a time every 2–4 weeks (except MRAs, up-titrated in 4–8 weeks). Can add a medicine before already-commenced medicines have been up-titrated to maximum tolerated dose. |
Start each medicine as soon as clinically possible, including:
|
|
| Congested patients | |||
First step |
(or ARB if not tolerated |
(or ACE inhibitor if problematic hypotension and then consider switching to ARNI later), and
| |
| Second step |
|
| |
| Once euvolaemic |
|
| |
| Euvolaemic patients | |||
| First step |
(or ARB if not tolerated), and
|
(or ACE inhibitor; only if problematic hypotension, and then consider switching to ARNI later), and
| |
| Second step |
|
| |
b SGLT2 inhibitor is a single dose; doesn’t require up-titration
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; ARNI = angiotensin receptor neprilysin inhibitor; HFrEF = heart failure with reduced ejection fraction; MRA = mineralocorticoid receptor antagonist; SGLT2 = sodium–glucose co-transporter-2
Figure 1: HFrEF management algorithm, with one of several possible drug initiation regimens based on presence or absence of clinical congestion12
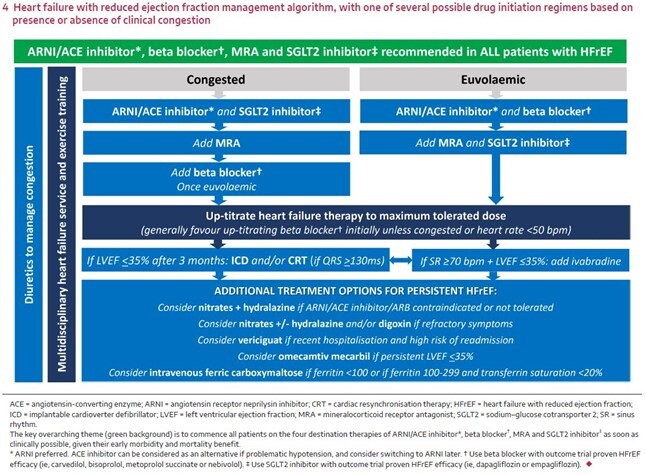
© Box 4 from Sindone AP, et al. Med J Aust. 2022 Aug 15;217:212-217.
Consensus statement on the current pharmacological prevention and management of heart failure.
This is an open access article under the terms of the Creative Commons Attribution-NonCommercial License
Additional guidance for standard treatment in the 2022 consensus statement:12
- Up-titrating a heart failure beta blocker is generally preferred initially, unless the patient is congested or their pulse is < 50 beats per minute.
- An ARNI (or ACE inhibitor) is preferred over an ARB because none of the heart failure ARB studies demonstrated a reduction in all-cause mortality.
- If patients are already taking an ACE inhibitor or ARB, replace it with an ARNI (after a 36-hour washout period for ACE inhibitor).
- The benefits of ARNIs and SGLT2 inhibitors are seen early, making a strong case for starting them upfront before full titration of other medicines already started, which may mean starting more than one medicine simultaneously.
- The recommended standard treatment for HFmrEF is similar to HFrEF: an ARNI (or ACE inhibitor or ARB), heart failure beta blocker and MRA up-titrated to the maximum tolerated dose, and an SGLT2 inhibitor.
The 2022 consensus statement doesn’t include an algorithm for HFmrEF nor specific guidance on when and how to prescribe these medicines.
PBS listings and 2022 consensus statement for standard treatment
There are some differences between the PBS listings and 2022 consensus statement recommendations that are relevant to prescribing PBS-subsidised vericiguat.1,12
Standard treatment
The main difference is regarding standard treatment. The PBS listing for vericiguat requires a patient to be treated with standard treatment which must include at least two medicines: a beta blocker and ARNI or ACE inhibitor or ARB (unless contraindicated or intolerant). The 2022 consensus statement includes four medicines as standard treatment: an ARNI (or ACE inhibitor), heart failure beta blocker, MRA and SGLT2 inhibitor.1,12 See Table 3 for specific PBS-subsidised heart failure medicines.
The Pharmaceutical Benefits Advisory Committee (PBAC) recognised at its March 2022 PBAC Meeting when considering the listing of vericiguat that four medicines – beta blockers, ARNIs or ACE inhibitors or ARBs, MRAs and SGLT2 inhibitors – and also diuretics would be used as ‘standard of care’.15
Table 3: Use of MRAs, ARNIs and SGLT2 inhibitors for the treatment of heart failure: comparison of PBS- subsided restrictions with 2022 consensus statement recommendations
|
Heart failure medicine |
PBS restriction |
Each medicine’s PBS-subsided restrictions compared with 2022 consensus statement recommendations |
|
MRA (spironolactone) |
Unrestricted Benefit |
|
|
Authority Required (STREAMLINED) |
There are exceptions:16 a) Permitted before a beta blocker if this is recommended by ‘local guidelines’. b) Permitted without first being first stabilised on an ACE inhibitor (or ARB) if these medicines are contraindicated according to the TGA-approved Product Information or cannot be tolerated. |
|
|
SGLT2 inhibitors (dapagliflozin or empagliflozin) |
Authority Required (STREAMLINED) |
|
c unless (i) a contraindication is listed in the Product Information or (ii) there is an existing/expected intolerance
Optimal
Another difference is the 2022 consensus statement doesn’t use the term ‘optimal’ that is used in the PBS listings. It states that the four medicines for standard treatment should be up-titrated to their maximum tolerated doses as soon as clinically possible.1,12
Acute versus decompensated heart failure
The definitions of heart failure in the 2021 international update state that the preferred term for patients who need to be hospitalised is ‘decompensated heart failure’.11
Patients who present with rapid onset or progressively escalating symptoms, and/or signs of heart failure that are associated with adverse outcomes, requiring urgent evaluation and treatment are characterised as either:11
- acutely decompensated due to an inciting event (eg, atrial fibrillation with rapid ventricular response) or
- chronically and progressively worsening with marked deterioration of heart failure signs and symptoms despite ongoing therapy, who require urgent intervention, hospitalisation or rapid escalation of therapies, including advanced therapies.
‘Persistent HFrEF’ rather than ‘stable HFrEF’
The 2021 international update states that it’s not only important to be clear about the patient’s current clinical status at a point in time, but also the direction in which the patient’s clinical status is headed. This is critical for determining whether to continue with the same treatment or change treatment.11
In particular, patients with HFrEF whose condition has not worsened may be erroneously regarded as being ‘stable’. (The use of ‘stable’ here is different to ‘stabilisation’ that applies to patients after a decompensation event) .11
There is evidence that improvement can be achieved for these patients by further optimising management with add-on therapies for HFrEF.11
When a patient has a baseline LVEF ≤ 40%, and then has a 10-point increase from baseline and a second measurement that is > 40%, their condition can be classified as ‘improved HFrEF’. These patients should continue receiving the same treatment. including optimised standard treatment.13
Patients with HFrEF and LVEF that remains ≤ 40% in response to optimal standard treatment, and who have ongoing symptoms/signs and/or limited functional capacity, may be more accurately described as showing a lack of improvement. Rather than being stable, they can be classified as having ‘persistent HFrEF’.11,13
It is recommended that patients with ‘persistent HFrEF’ should start add-on therapies.12,13
Management following discharge from hospital
The most vulnerable period for patients with heart failure is the first few weeks after discharge from hospital.10 At 30 days after hospitalisation for a decompensation event the rate of all-cause readmission is 20% and all-cause mortality is 8%.17
Patients with heart failure should be reviewed 7–14 days after discharge to assess fluid status and that standard treatment medicines are at target or maximum tolerated doses. If they are not, they should be up-titrated as soon as practical.10,18
Vericiguat for heart failure
The 2022 consensus statement identifies sub-groups of patients with persistent HFrEF.11-13
It recommends vericiguat as add-on therapy to standard treatment for the sub-group of patients who have a history of recent hospitalisation and high risk of readmission.12
The risk of readmission is higher for patients whose heart failure is inadequately controlled at the initial admission, when causes of the heart failure are not corrected, related comorbidities not addressed, insufficient support when moving back to the community, difficulties in self-management and social deprivation.19
The PBS listing for vericiguat differs from the 2022 consensus statement, using LVEF < 45% as the cut-off level, and is more specific about the clinical criteria regarding recent worsening and stabilisation after a decompensation event.1
This means that patients classified as having HFmrEF (defined as LVEF 41–49%) according to the current guidance who have an LVEF < 45% (and meet all the other eligibility criteria) may be prescribed PBS-listed vericiguat.1,12
What is the key study evidence?
VICTORIA trial
The VICTORIA trial was a phase 3 randomised, double-blind, placebo-controlled trial. It enrolled 5050 people (average age 67 years; 76% male) with symptomatic (NYHA class II–IV) heart failure and LVEF < 45% within 12 months before randomisation (mean LVEF 28.9%).20
At baseline the use of standard treatment was high, with 60% of people taking three medicines: a beta blocker, MRA and ARNI or ACE inhibitor or ARB. For specific medicines, 93% of patients were taking a beta blocker, 73% an ACE inhibitor or ARB, 70% an MRA, 15% an ARNI and 3% an SGLT2 inhibitor. At randomisation 67% of patients had been hospitalised in the previous 3 months,17% 3– 6 months ago, and 16% had received IV diuretic therapy within 3 months.2,20
The primary outcome was time to first occurrence of hospitalisation for heart failure or cardiovascular death.20
The relative hazard of this outcome was reduced by 10% compared to placebo (35.5% with vericiguat vs 38.5% with placebo) over the median follow-up time of 10.8 months.15,20 The 3% absolute risk reduction (ARR) corresponded to an absolute event-rate reduction of 4.2 events per 100 patient-years, resulting in a number needed to treat of approximately 24 patients to prevent a primary outcome event (treatment with vericiguat for 1 year).20
The reduction in the primary outcome was mostly driven by the relative hazard of the time to first hospitalisation for heart failure, which was reduced by 10% (27.4% with vericiguat vs 29.6% with placebo) over the median follow-up time of 10.8 months. The relative hazard of the time to cardiovascular death (16.4% with vericiguat vs 17.5% with placebo) over the median follow-up time of 10.8 months was not statistically different.9,15,20
Reason for PBS listing
At the July 2022 PBAC Meeting, vericiguat for chronic heart failure was recommended for listing on the PBS.21
Based on the VICTORIA trial, the key study in the listing submission, the PBAC considered that the claim of superior comparative effectiveness was reasonable.21
It agreed there was a clinical need for a treatment with a different mechanism of action in a specific, narrowly defined group of high-risk patients with acute decompensated heart failure who are stable and euvolaemic.15
However, the PBAC found that the magnitude of benefit associated with vericiguat plus standard treatment in the VICTORIA trial was modest, based on the ARR of 3%. It noted that this was similar in magnitude to recent trials of dapagliflozin and empagliflozin for HFrEF (LVEF ≤ 40%) that had an ARR of approximately 5%, though the VICTORIA trial had a higher risk population.15,21
The PBAC noted there were key differences between the trial population and the likely PBS population in terms of baseline N-terminal pro hormone B-type natriuretic peptide (NT-proBNP), age, gender, eGFR and concomitant medicines. This suggests that the likely PBS population may include patients with more severe disease, who may not achieve the same benefits associated with vericiguat observed in the VICTORIA trial.15
Given the clinical need was in a small group of high-risk patients, the PBAC proposed the PBS listing should restrict use to patients who match the VICTORIA trial population as much as practical and with initiation limited to cardiologists.15
It also noted that the patient population in the VICTORIA trial had limited exposure to an ARNI and SGLT2 inhibitors. It suggested that when adding vericiguat for people already receiving standard treatment that included an ARNI and SGLT2 inhibitor, the degree of benefit was unknown.15
The PBAC considered that the claim of comparable safety was reasonable. It noted a slight increase in drug-related adverse events compared with placebo (14.6% versus 11.7% respectively).15
Dosing issues
Compared to other treatments for heart failure, vericiguat only requires once-daily dosing.4
The recommended:2
- start dose is 2.5 mg once daily
- target maintenance dose is 10 mg once daily.
The start dose should be doubled approximately every 2 weeks to reach the target maintenance dose, as tolerated by the patient.2
The pack size for all PBS-listed doses of vericiguat (2.5 mg, 5 mg and 10 mg) is 28 tablets.1
Food increases the bioavailability of vericiguat.22 It should be taken orally once daily with food. For patients with trouble swallowing tablets whole, vericiguat may be crushed and mixed with water immediately before taking.2
If the patient experiences symptomatic hypotension, consider dose adjustment of concomitant diuretics and treatment of other causes of hypotension (such as hypovolaemia). Consider temporary dose reduction or cessation of vericiguat if symptomatic hypotension persists despite such measures.2
No dosage adjustments are required for:2
- patients with eGFR ≥ 15 mL/min/1.73 m2 (without dialysis)
- patients with mild or moderate hepatic impairment
- older people.
Safety issues
For information about reporting adverse reactions to the TGA, or to report suspected adverse reactions online, see the TGA website.
Drug interactions
Vericiguat’s low potential for pharmacokinetic interactions make it well-suited for patients with heart failure with multiple comorbidities and multiple concomitant medicines.22
Concomitant use of vericiguat and phosphodiesterase type 5 (PDE5) inhibitors such as sildenafil is not recommended due to the potential for increased risk of symptomatic hypotension.2
Vericiguat is contraindicated for patients with concomitant use of other sGC stimulators, such as riociguat.2
Pregnancy and breastfeeding
Vericiguat is not recommended during pregnancy (Category D) based on animal studies. People of childbearing potential should use effective forms of contraception during treatment with vericiguat.2
There is no evidence on the effect of vericiguat on human lactation. Vericiguat has been found in the milk of lactating rats and as such, a risk to the breastfed child cannot be excluded. A decision must be made whether to stop breastfeeding or stop vericiguat, considering the benefit of breastfeeding and vericiguat therapy.2
Side effects
Results from the VICTORIA trial showed that the overall side effects were similar for treatment and placebo groups.20 Common side effects (≥ 1%) that occurred in higher rates in the treatment group compared to the placebo group include:2,20
- hypotension
- anaemia
- nausea
- dyspepsia
- vomiting
- gastroesophageal reflux disease
- dizziness
- headache.
Routine testing and monitoring
As vericiguat is well tolerated with minimal haemodynamic impact, it does not require routine laboratory testing or therapeutic drug monitoring.4
Information for patients
Prescribers and pharmacists are encouraged to discuss the following with patients and carers:2,23
- Take one tablet at the same time each day with food. If you miss a dose, take it as soon as you remember on the same day of the missed dose. If you remember the next day, skip the dose you missed. Do not take two doses on the same day.
- You will normally start by taking 2.5 mg once a day for the first 2 weeks. Then you will increase the dose every 2 weeks. The normal target maintenance dose is 10 mg once a day.
- Contact your GP or pharmacist if you cannot swallow the tablet whole to find out how to take the medicine.
- Most side effects are minor and temporary. Call your GP if you have signs of low blood pressure. This can be feeling dizzy or lightheaded. Report any side effects to the TGA.
- Do not drive or use machines until you know how this medicine affects you.
- Tell your GP or pharmacist if you drink alcohol. The effects with this medicine are unknown.
- If you are of childbearing age, you will need to use an effective form of contraception while taking this medicine.
- This medicine contains lactose. Let your GP or pharmacist know if you are intolerant to lactose.
More information
- NPS MedicineWise Heart failure: taking an active role
- NPS MedicineWise RADAR articles: Dapagliflozin (Forxiga) and Empagliflozin (Jardiance) for heart failure with reduced ejection fraction (LVEF ≤ 40%)
- Sindone AP, et al. Consensus statement on the current pharmacological prevention and management of heart failure. Med J Aust. 2022 Aug 15;217:212-217
- 2022 AHA/ACC/HFSA Guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines
- 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure
References
- Pharmaceutical Benefits Scheme. PBS Schedule: Summary of Changes (December 2022). Canberra: Australian Government Department of Health, 2022 (accessed 1 December 2022).
- Bayer Australia Ltd. Vericiguat (Verquvo) product information. Pymble, NSW: Bayer Australia Ltd, 2021 (accessed 11 October 2022).
- Sandner P, Follmann M, Becker-Pelster E, et al. Soluble GC stimulators and activators: Past, present and future. Br J Pharmacol 2021.
- Kassis-George H, Verlinden NJ, Fu S, et al. Vericiguat in heart failure with a reduced ejection fraction: Patient selection and special considerations. Ther Clin Risk Manag 2022;18:315-22.
- Bayer Australia Ltd. Riociguat (Adempas) product information. Pymble, NSW: Bayer Australia Ltd, 2022 (accessed 11 October 2022).
- Pharmaceutical Benefits Scheme. PBS Section 100 - Highly Specialised Drugs Program. Canberra: Australian Government Department of Health and Aged Care, 2019 (accessed 11 October 2022).
- Kansakar S, Guragain A, Verma D, et al. Soluble guanylate cyclase stimulators in heart failure. Cureus 2021;13:e17781.
- Hulot JS, Trochu JN, Donal E, et al. Vericiguat for the treatment of heart failure: mechanism of action and pharmacological properties compared with other emerging therapeutic options. Expert Opin Pharmacother 2021;22:1847-55.
- Therapeutic Goods Administration. Australian Public Assessment Report for Verquvo. Canberra: Australian Government Department of Health and Aged Care, 2022 (accessed 11 October 2022).
- Atherton JJ, Sindone A, De Pasquale CG, et al. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: Guidelines for the prevention, detection, and management of heart failure in Australia 2018. Heart Lung Circ 2018;27:1123-208.
- Bozkurt B, Coats AJS, Tsutsui H, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail 2021;23:352-80.
- Sindone AP, De Pasquale C, Amerena J, et al. Consensus statement on the current pharmacological prevention and management of heart failure. Med J Aust 2022;217:212-7.
- Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022;145:e895-e1032.
- McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599-726.
- Pharmaceutical Benefits Scheme. Public Summary Document: Vericiguat (March 2022 PBAC Meeting). Canberra: Australian Government Department of Health and Aged Care, 2022 (accessed 11 October 2022).
- Pharmaceutical Benefits Scheme. PBS Schedule: December 2022. Canberra: Australian Government Department of Health and Aged Care, 2022 (accessed 1 December 2022).
- Al-Omary MS, Davies AJ, Evans TJ, et al. Mortality and Readmission Following Hospitalisation for Heart Failure in Australia: A Systematic Review and Meta-Analysis. Heart Lung Circ 2018;27:917-27.
-
Audehm R. Vericiguat (Verquvo) for persistent heart failure with LVEF < 45%. Personal Communication. 2 November 2022.
- Marwick T, Magliano D, Shaw J, et al. Change of heart: Time to end cardiovascular complacency. Melbourne: Baker IDI Heart and Diabetes Institute, 2016 (accessed 7 November 2022).
- Armstrong PW, Pieske B, Anstrom KJ, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med 2020;382:1883-93.
- Pharmaceutical Benefits Scheme. Public Summary Document: Vericiguat (July 2022 PBAC Meeting). Canberra: Australian Government Department of Health and Aged Care, 2022 (accessed 11 October 2022).
- Boettcher M, Gerisch M, Lobmeyer M, et al. Metabolism and pharmacokinetic drug-drug interaction profile of vericiguat, a soluble guanylate cyclase stimulator: Results from preclinical and phase I healthy volunteer studies. Clin Pharmacokinet 2020;59:1407-18.
- Bayer Australia Ltd. Vericiguat (Verquvo) consumer medicine information. Pymble, NSW: Bayer Australia Ltd, 2021 (accessed 11 October 2022).
