What is in this leaflet
This leaflet answers some common questions about this medicine. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking this medicine against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine.
You may need to read it again.
What this medicine is used for
The name of your medicine is APO-Valsartan. It contains the active ingredient valsartan.
This medicine belongs to a group of medicines called angiotensin II receptor antagonists (AIIRAs).
It is used to treat:
Hypertension
This medicine is used to control high blood pressure, also called hypertension. Everyone has blood pressure. This pressure helps get your blood around your body. Your blood pressure may be different at different times of the day, depending on how busy or worried you are. You have hypertension when your blood pressure stays higher than is needed, even when you are calm and relaxed.
There are usually no symptoms of hypertension. The only way of knowing that you have it is to have your blood pressure checked regularly.
High blood pressure increases the workload of the heart and blood vessels. If it continues for a long time, it can damage the blood vessels in the brain, heart and kidneys. This can lead to stroke, heart failure or kidney failure. High blood pressure increases the risk of heart attacks. Lowering your blood pressure reduces the chance of these disorders happening.
Heart Failure
This medicine is used to treat heart failure. Heart failure means that the heart muscle cannot pump blood strongly enough to supply all the blood needed throughout the body. Heart failure is not the same as heart attack and does not mean that the heart stops. This medicine helps the heart to function better and relieves some of the symptoms of heart failure.
Heart Attack
This medicine is also used to treat people after they have had a heart attack (myocardial infarction), to reduce the risk of further heart damage and reduce further heart problems.
Ask your doctor if you have any questions about why valsartan has been prescribed for you. Your doctor may have prescribed it for another reason.
This medicine is not addictive.
This medicine is available only with a doctor's prescription.
Use in children
There is not enough information to recommend the use of valsartan in children (below 18 years of age).
This medicine should not be used in children.
Before you take this medicine
When you must not take it
Do not take this medicine if you have an allergy to:
- valsartan or any of the ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin
- fainting or hayfever-like symptoms
Do not take valsartan if you are pregnant or intend to become pregnant. Valsartan may affect your developing baby if you take it during pregnancy.
Do not take valsartan if you are also taking other blood pressure lowering medicines containing aliskiren and have type 2 diabetes.
Do not take valsartan if you have liver disease caused by blockage in the bile duct or any other severe liver disease. Valsartan is not recommended if you have these conditions.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking valsartan, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you have or have had any of the following medical conditions:
- heart disease, heart failure or high blood pressure that is being treated with large doses of diuretics (also called water or fluid tablets), or are being treated with beta-blockers, aliskiren and/ or ACE-inhibitors
- high blood pressure due to narrowing of the arteries in the kidney
- any other kidney problems or are having dialysis
- milder forms of liver disease
- a hormonal disorder called primary hyperaldosteronism (Conn's syndrome), which can increase blood pressure
- swelling, mainly of the face and throat, while taking other medicines (including an ACE inhibitor or aliskiren)
- you have recently had severe vomiting or diarrhoea
- you are severely limiting your salt intake.
- obstructed blood flow through the heart from narrowing of valves (stenosis) or enlarged septum of the heart (HOCM)
Tell your doctor if you are breastfeeding or plan to breastfeed. It is not known if valsartan, the active ingredient, passes into the breast milk and could affect your baby.
If you have not told your doctor about any of the above, tell them before you start taking this medicine.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and valsartan interfere with each other. These include:
- ACE-inhibitors or aliskiren, which are also medicines used to treat hypertension or other heart conditions
- beta-blockers, which are medicines used to treat hypertension or other heart conditions.
- some diuretics (water or fluid pills) e.g. spironolactone, triamterene, amiloride
- a class of anti-inflammatory medicines called NSAIDs and COX-2 inhibitors
- potassium supplements, salt substitutes containing potassium, or other medications that may increase potassium levels (such as heparin)
- some antibiotics (rifampicin), anti-rejection drugs (cyclosporin), antiretrovirals (ritonavir)
- lithium used to treat conditions such as bipolar or schizophrenia.
If you are taking any of these, you may need a different dose, or you may need to take different medicines.
Your doctor may also check your kidney function.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking valsartan.
How to take this medicine
Follow all directions given to you by your doctor carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the label, ask your doctor or pharmacist for help.
How much to take
For hypertension, the usual dose is one 80 mg tablet once a day. If your blood pressure is still too high after 4 weeks, your doctor may increase the dose to 160 mg once a day, or from 160 mg to 320 mg once a day. If your blood pressure is still too high, your doctor may add a different type of blood pressure lowering medicine.
For heart failure, the usual starting dose is 40 mg twice daily. Your doctor may increase the dose gradually up to one 160 mg tablet twice daily.
Following a heart attack, treatment is generally started at a dose of 20 mg (half a 40 mg tablet) twice daily. Your doctor may increase the dose gradually up to 160 mg twice daily.
Do not stop taking your medicine or change your dosage without first checking with your doctor.
How to take it
Swallow the tablets with a full glass of water.
It does not matter if you take it after food or on an empty stomach, as long as you take it the same way each day.
If your stomach is upset after taking valsartan, always take it after a meal (e.g. breakfast).
When to take it
Take your medicine at about the same time each day. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
How long to take it for
Continue taking your medicine for as long as your doctor tells you.
Valsartan helps to control your condition but does not cure it. It is important to keep taking your medicine even if you feel well.
It will take at least 4 weeks for this medicine to have its full effect. After that, it will be continued for as long as your doctor thinks is needed.
If you forget to take it
If it is almost time to take your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take it as soon as you remember, and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for the dose that you missed. This may increase the chance of you experiencing side effects.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much of this medicine. Do this even if there are no signs of discomfort or poisoning.
Too much of valsartan may make you feel dizzy, lightheaded or faint. You may experience rapid, shallow breathing or cold, clammy skin. Your heartbeat may be faster than usual.
This is because your blood pressure is too low.
While you are taking this medicine
Things you must do
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking valsartan.
Tell any other doctors, dentists, and pharmacists who treat you that you are taking valsartan.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking valsartan. It may affect other medicines used during surgery.
If you become pregnant while taking valsartan, tell your doctor immediately. You should not take this medicine while you are pregnant.
Tell your doctor if, for any reason, you have not taken valsartan exactly as prescribed. Otherwise your doctor may think that it was not effective and change your treatment unnecessarily.
If you are about to have any blood tests, tell your doctor that you are taking valsartan. It may interfere with the results of some tests.
Keep all of your doctor's appointments so that your progress can be checked. Do this even if you feel well.
It is important to keep track of your progress. Your doctor will want to check your blood pressure and your kidney and liver function from time to time.
Things you must not do
Do not take valsartan to treat any other complaints unless your doctor tells you to.
Do not give valsartan to anyone else, even if they have the same condition as you.
Do not stop taking valsartan or lower the dosage without checking with your doctor.
Things to be careful of
Be careful when driving or operating machinery or doing jobs that require you to be alert while you are taking valsartan until you know how this medicine affects you. This medicine can cause tiredness, sleepiness or dizziness in some people. If you have these symptoms, do not drive or do anything else that could be dangerous.
If this medicine makes you feel dizzy or light-headed, be careful when getting up from a sitting or lying position. Dizziness can usually be prevented by getting up slowly and flexing leg muscles and toes to get the blood flowing. When getting out of bed, dangle your legs over the side for a minute or two before standing up.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking valsartan.
All medicines can have side effects. Sometimes they are serious but most of the time they are not.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- headache
- dizziness, spinning sensation (vertigo)
- sleepiness, tiredness or weakness
- diarrhoea, constipation or wind
- nausea (feeling sick), vomiting
- loss of appetite, stomach pains or indigestion
- dry cough, sore throat or hoarse voice
- runny nose or congested sinuses
- more infections than usual
- pain in the back or joints
- muscle pain or cramps
- difficulty sleeping
- feeling anxious
- tingling or numbness in the hands or feet
- problems with sexual function.
Tell your doctor immediately or go to the Accident and Emergency department at your nearest hospital if you notice any of the following:
- signs of allergy such as cough, shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue, or other parts of the body; rash, itching or hives on the skin; fainting or hayfever-like symptoms
- feeling of fast or irregular heart beat (pounding, racing, skipping beats)
- chest pain
- shortness of breath not caused by exercise, with swelling of legs or feet
- tiredness or lack of energy, being short of breath when exercising, dizziness and looking pale
- constant "flu-like" symptoms such as chills, fever, sore throat, aching joints, sores in mouth, swollen glands
- severe dizziness or fainting
- liver disease with nausea, vomiting, loss of appetite, feeling generally unwell, fever, itching, yellowing of the skin and eyes, and dark coloured urine
The above list includes serious side effects that may require medical attention.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Other side effects not listed above may also occur in some people.
Storage and disposal
Storage
Keep your medicine in its original packaging until it is time to take it. If you take your medicine out of its original packaging it may not keep well.
Keep your medicine in a cool dry place where the temperature will stay below 25°C.
Do not store this medicine or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep this medicine where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What APO-Valsartan looks like
40 mg tablets: Yellow, modified, capsule shaped, film-coated tablets, engraved 'APO' on one side and 'VA' scored '40' on the other side. AUST R 185806. AUST R 185853.
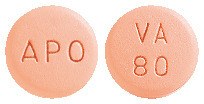
80 mg tablets: Pale red, round, biconvex, film-coated tablets, engraved 'APO' on one side and 'VA' over '80' on the other side. AUST R 185814. AUST R 185854.
160 mg tablets: Yellow, modified, capsule shaped, film-coated tablets, engraved 'APO' on one side and 'VA160' on the other side. AUST R 185821. AUST R 185855.
320 mg tablets: Dark grey-violet, oval shaped, film-coated tablets, engraved 'APO' on one side and 'VA320' on the other side. AUST R 185826. AUST R 185856.
Blister packs of 7, 14, 28, 30, 56 tablets
Bottle packs of 28, 100, 500 tablets
Ingredients
Each tablet contains 40, 80, 160 or 320 mg of valsartan as the active ingredient.
It also contains the following inactive ingredients:
- powdered cellulose
- calcium hydrogen phosphate dihydrate
- croscarmellose sodium
- colloidal anhydrous silica
- magnesium stearate
- hypromellose
- hyprolose
- macrogol 8000
- titanium dioxide
- iron oxide yellow iron oxide red purified water
- The 160 and 320 mg tablets also contain: iron oxide black.
This medicine is gluten-free, lactose-free, sucrose-free, tartrazine-free and free of other azo dyes.
Sponsor
Apotex Pty Ltd
16 Giffnock Avenue
Macquarie Park, NSW 2113
APO and APOTEX is a registered trade mark of Apotex Inc.
This leaflet was last updated in: July 2019.
Published by MIMS September 2019






 Since this was a trial with an active control (captopril), an additional analysis of all-cause mortality was performed to estimate how valsartan would have performed versus placebo. Using the results of the previous reference myocardial infarction trials - SAVE, AIRE, and TRACE - the estimated effect of valsartan preserved 99.6% of the effect of captopril (97.5% CI = 60-139%). Combining valsartan with captopril did not add further benefit over captopril alone, therefore this combination is not recommended. There was no difference in all-cause mortality based on age, gender, race, baseline therapies or underlying disease.
Since this was a trial with an active control (captopril), an additional analysis of all-cause mortality was performed to estimate how valsartan would have performed versus placebo. Using the results of the previous reference myocardial infarction trials - SAVE, AIRE, and TRACE - the estimated effect of valsartan preserved 99.6% of the effect of captopril (97.5% CI = 60-139%). Combining valsartan with captopril did not add further benefit over captopril alone, therefore this combination is not recommended. There was no difference in all-cause mortality based on age, gender, race, baseline therapies or underlying disease.
 Other adverse experiences that occurred in controlled clinical trials of patients treated with valsartan (> 0.2% of valsartan patients) are listed below. It cannot be determined whether these events were causally related to valsartan. See Table 3.
Other adverse experiences that occurred in controlled clinical trials of patients treated with valsartan (> 0.2% of valsartan patients) are listed below. It cannot be determined whether these events were causally related to valsartan. See Table 3. Other reported events seen less frequently in clinical trials included chest pain, syncope, anorexia and vomiting.
Other reported events seen less frequently in clinical trials included chest pain, syncope, anorexia and vomiting.