What is in this leaflet
This leaflet answers some common questions about Axit. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking Axit against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with your medicine. You may need to read it again.
What Axit is used for
Axit is used in the treatment of depression including relapse prevention.
Depression is longer lasting or more severe than "low moods" everyone has from time to time due to the stress of everyday life. It is thought to be caused by a chemical imbalance in parts of the brain. This affects your whole body and can cause emotional and physical symptoms such as feeling low in spirit, loss of interest in activities, unable to enjoy life, poor appetite or overeating, disturbed sleep, often waking up early, loss of sex drive, lack of energy and feeling guilty over nothing.
This medicine corrects this chemical imbalance and may help relieve the symptoms of depression.
Your doctor, however, may prescribe it for another purpose.
Ask your doctor if you have any questions about why it has been prescribed for you.
This medicine is only available with a doctor's prescription.
Axit is not addictive.
Before you take Axit
When you must not take it
Do not take Axit:
- if you are allergic to medicines containing mirtazapine
- if you are allergic to any of the ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include skin rash, itching or hives, swelling of the face, lips, mouth, throat or other parts of the body, shortness of breath, wheezing or trouble breathing.
Do not take Axit if you are taking another medicine for depression called a monoamine oxidase inhibitor (MAOI) or have been taking an MAOI within the last 14 days. If you stop taking Axit, do not take MAOI during the next two weeks either. Taking Axit with an MAOI may cause a serious reaction with a sudden increase in body temperature, extremely high blood pressure and severe convulsions.
Examples of this type of medicine include phenelzine, tranylcypromine and selegiline.
Ask your doctor or pharmacist if you are not sure if you are or if you have been taking a MAOI medicine.
Do not take Axit if the packaging is torn or shows signs of tampering.
Do not take Axit if the expiry date printed on the pack has passed.
If you are not sure whether you should start taking Axit, talk to your doctor
Do not take Axit if you have galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption, as this medicine contains lactose.
Before you start to take it
Do not give Axit to a child or adolescent. The safety of Axit in patients under 18 years has not been established.
Tell your doctor if:
- you are allergic to any other medicines, foods, dyes or preservatives.
- you are pregnant or plan to become pregnant
Like most medicines of this kind, Axit is not recommended to be used during pregnancy. Your doctor will discuss the risks and benefits of taking Axit when pregnant.
- you are breastfeeding or wish to breastfeed.
It is not known whether Axit passes into breastmilk.
- if you have or have had any medical conditions, especially the following:
- thoughts of suicide or self-harm
- epilepsy (fits or convulsions)
- liver disease such as jaundice
- kidney disease
- heart disease
- low blood pressure
- certain kinds of heart conditions that may change your heart rhythm, a recent heart attack, heart failure, or take certain medicines that may affect the heart's rhythm.
- any mental illness (e.g. schizophrenia and manic depression)
- diabetes
- glaucoma (increased pressure in the eye)
- problems in urinating due to an enlarged prostate
- unexplainable high fever, sore throat and mouth ulcers
- galactose intolerance
- glucose-galactose malabsorption.
If you have not told your doctor about any of the above, tell them before you take Axit.
Tell your doctor if you react badly to lactose or milk before you start taking Axit. Axit tablets contain lactose.
Taking other medicines
Tell your doctor if you are taking any other medicines, including medicines you buy without a prescription from a pharmacy, supermarket or health food shop.
Some medicines may be affected by Axit or may affect how well it works. These include:
- other medicines (eg. SSRIs, venlafaxine, L-tryptophan, nefazodone) for depression, anxiety, obsessive compulsive disorders or pre-menstrual dysphoric disorder
- Monoamine Oxidase Inhibitors (such as tranylcypromine, phenelzine, and selegiline)
- medicines containing St. John's Wort (hypericum perforatum)
- phenytoin or carbamazepine, medicines used to treat epilepsy
- benzodiazepines, medicines used to treat anxiety and sleeping problems
- lithium, a medicine used to treat some psychiatric conditions
- methylene blue (used to treat high levels of methemoglobin in the blood)
- tramadol, a pain killer
- morphine, a medicine for severe pain
- cetirizine, a medicine for allergies
- warfarin, a medicine used to prevent blood clotting
- linezolid, erythromycin, an antibiotic
- rifampicin, a medicine used to treat tuberculosis
- medicines used to treat fungal infections such as ketoconazole
- HIV/AIDS medications
- cimetidine, a medicine used to treat reflux and stomach ulcers
- triptans such as sumatriptan, naratriptan and zolmitriptan, medicines used to treat migraine
- medicines that may affect the heart's rhythm such as certain antibiotics and some anti-psychotics.
Your doctor can tell you what to do if you are taking any of these medicines.
Your doctor and pharmacist may have more information on medicines to be careful with or avoid while taking Axit
If you are not sure whether you are taking any of these medicines, check with your doctor or pharmacist.
How to take Axit
Follow all directions given to you by your doctor and pharmacist carefully. They may differ from the information contained in this leaflet.
How much to take
Your doctor will tell you how much Axit to take each day. Take exactly the amount your doctor tells you.
The usual starting dose is 15 mg per day. Your doctor may slowly increase this dose depending on how you respond to this medicine. The effective dose for most people is usually between 30 and 45 mg daily.
Your doctor may have prescribed a different dose.
If you do not understand the instructions on the box, ask your doctor or pharmacist for help.
When to take it
Take your medicine at about the same time each day.
Your doctor will tell you when to take your tablets.
The tablet(s) should be taken at the same time each day, preferably as a single night-time dose before going to bed; if recommended by your doctor, Axit may be taken in sub-doses equally divided over the day (once in the morning and once at night-time before going to bed).
How to take it
Swallow the tablet(s), without chewing, together with some water or other fluid.
Do not crush or chew the tablets.
Axit 15 and Axit 30 tablets can be divided in half along the breakline, if advised by your doctor or pharmacist.
Axit can be taken with or without food.
How long to take it
Keep taking Axit until your doctor tells you to stop.
For depression, the length of treatment will depend on how quickly your symptoms improve. Most antidepressants take time to work, so do not be discouraged if you don't feel better right away. Some of your symptoms may improve in 1 to 2 weeks but it can take up to 2 - 4 weeks longer to feel the full benefit of the medicine.
Even when you feel well, you will usually have to take Axit for 4 to 6 months or even longer to make sure the benefits will last.
If you forget to take it
ONCE DAILY DOSING
If you forget to take the tablet before you go to bed, do not take the missed dose the next morning. It may cause drowsiness or sleepiness during the day. Continue treatment in the evening with your normal dose.
TWICE DAILY DOSING
- Morning dose forgotten - simply take it together with your evening dose.
- Evening dose forgotten - do not take it with the next morning dose. Continue treatment with your normal morning and evening doses.
- Both doses forgotten - do not try to make up for the missed tablets. Continue with your usual morning and evening dose the next day.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital, if you think you or anyone else may have taken too much Axit. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
If you take too many Axit tablets, you may feel drowsy, dizzy, confused and agitated.
You may also have changes to your heart rhythm (fast, irregular heartbeat) and/or fainting which could be symptoms of a life-threatening condition known as Torsades de Pointes.
While you are taking Axit
Things you must do
Tell your doctor immediately if you develop fever, chills, sore throat or mouth ulcers or other signs of frequent infections. Stop taking Axit and consult with your doctor for a blood test. In rare cases Axit can cause disturbances in the production of blood cells (bone marrow depression). Some people become less resistant to infection because Axit can cause a temporary shortage of white blood cells (granulocytopenia). In rare cases Axit can also cause a shortage of red and white blood cells, as well as blood platelets (aplastic anaemia), a shortage of blood platelets (thrombocytopenia) or an increase in the number of white blood cells (eosinophilia). While rare, these symptoms most commonly appear after 4 - 6 weeks of treatment.
Tell your doctor immediately or go to the nearest hospital for treatment if you have any suicidal thoughts or other mental/mood changes. Occasionally, the symptoms of depression or other psychiatric conditions may include thoughts of harming yourself or committing suicide. Until the full antidepressant effect of your medicine becomes apparent, it is possible these symptoms may increase in the first few weeks of treatment.
Information from clinical trials have shown an increased risk of suicidal behaviour in young adults aged less than 25 years with psychiatric conditions who were treated with an antidepressant.
If you or someone you know is showing warning signs of suicide-related behaviour while taking Axit, contact your doctor or a mental health professional right away or go to the nearest hospital for treatment. These signs include:
- thoughts or talk about death or suicide
- thoughts or talk of self-harm or harm to others
- any recent attempts of self harm
- increase in aggressive behaviour, irritability or agitation.
All mentions of suicide or violence must be taken seriously.
You may find it helpful to tell a relative or close friend that you are depressed and ask them to read this leaflet. You might ask them to tell you if they think your depression is getting worse, or if they are worried about changes in your behaviour.
Tell your doctor if you become pregnant while taking this medicine. Do not stop taking your tablets until you have spoken to your doctor.
If you use Axit until, or shortly before birth, your baby should be supervised for possible adverse effects.
Ask your doctor whether you can breastfeed, while taking Axit.
Tell your doctor if, for any reason, you have not taken your medicine exactly as prescribed. Otherwise, your doctor may think that it was not effective and change your treatment unnecessarily.
Tell your doctor if you feel the tablets are not helping your condition.
Be sure to keep all of your appointments with your doctor so that your progress can be checked. You may need to have blood tests from time to time.
Before starting any new medicine, tell your doctor or pharmacist that you are taking Axit.
Tell all the doctors, dentists and pharmacists who are treating you that you are taking Axit.
Things you must not do
Do not drive or operate machinery until you know how Axit affects you. Axit may cause drowsiness, dizziness or sleepiness in some people and affect alertness and concentration. If any of these occur, do not drive, operate machinery or do anything else that could be dangerous.
Do not suddenly stop taking Axit, or lower the dose, without first checking with your doctor.
Do not let yourself run out of medicine over weekends or on holidays.
Do not stop taking Axit, even if you feel better, unless advised by your doctor. Suddenly stopping Axit may cause nausea (feeling sick), headache, dizziness, anxiety, agitation.
Your doctor may want you to gradually reduce the amount of Axit you are taking before stopping completely.
Do not use Axit to treat any other conditions unless your doctor tells you to.
Do not give Axit to anyone else, even if their symptoms seem similar to yours or if they have the same condition as you.
Things to be careful of
You are advised not to drink any alcohol while taking Axit. Combining Axit and alcohol can make you more sleepy and less alert. Your doctor may suggest you avoid alcohol while being treated with this medicine.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Axit.
Axit helps most people with depression, but it may have unwanted side effects in a few people. All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Ask your doctor or pharmacist to answer any questions you may have.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Tell your doctor if you notice any of the following and they worry you:
- lethargy, drowsiness or sleepiness
- headache
- tiredness
- increase in appetite and weight gain
- dry mouth
- nausea, vomiting
- diarrhoea
- constipation
- dizziness
- dizziness or faintness when getting up quickly from a lying or sitting position (low blood pressure)
- abnormal sensations in the mouth, sensations of numbness in the mouth or swelling in the mouth
- aggression
- swollen ankles or feet as a result of fluid accumulation (oedema)
- rash or skin eruptions
- nightmares/vivid dreams
- tingling fingers or toes
- painful joints
- back pain
- muscle aches and pains
- restless legs
- abnormal sensation in the skin for example burning, stinging, tickling or tingling
- urge to move
- speech disorders
- difficulty in passing urine (urinary retention)
- anxiety, insomnia. These may be symptoms of depression
- increased prolactin hormone levels in blood (hyperprolactinaemia, including symptoms such as enlarged breasts and/or milky nipple discharge)
- sleepwalking
- prolonged painful erection of the penis
Tell your doctor immediately or go to Accident and Emergency at the nearest hospital if you notice any of the following:
- suicidal ideation or behaviour
- epileptic attack (seizures)
- shaking or tremors
- sudden muscle contractions (myoclonus)
- attack of excessive excitability (mania)
- agitation
- confusion
- hallucinations
- changes to your heart rhythm
- fainting
- yellow colouring of eyes or skin; this may suggest disturbance in liver function
- abdominal pain and nausea; this may suggest inflammation of the pancreas
- generalised fluid retention with weight gain
- skin rash, itching or hives; swelling of the face, lips or tongue which may cause difficulty breathing
- severe skin reactions
- signs of infection such as sudden unexplainable high fever, sore throat and mouth ulcers
- a combination of symptoms such as fever, sweating, increased heart rate, diarrhoea, (uncontrollable) muscle contractions, shivering, overactive reflexes, restlessness, mood changes, unconsciousness and increased salivation (serotonin syndrome)
- muscle pain, stiffness and/or weakness, darkening or discolouration of the urine (rhabdomyolysis)
Other side effects not listed above may also occur in some patients.
Tell your doctor if you notice anything else that is making you feel unwell.
Ask your doctor or pharmacist if you don't understand anything in this list.
After taking Axit
Storage
Keep your tablets in their blister pack until it is time to take them The tablets may not keep as well if you take them out of the blister pack.
Store below 30°C in a dark, dry place.
Do not store Axit or any other medicine in the bathroom or near a sink. Heat and dampness will destroy some medicines.
Keep this medicine where young children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
Return any unused medicine to your pharmacist.
Medicines should not be disposed of via waste water or household waste.
Product description
What it looks like
Axit 15 tablets are round, yellow, film-coated tablets marked "MR|15" on one side and "G" on the other.
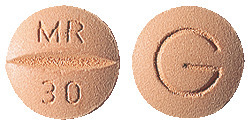
Axit 30 tablets are round, buff coloured, film-coated tablets marked "MR|30" on one side and "G" on the other.
Axit 45 tablets are round, white, film-coated tablets marked with "MR 45" on one side and "G" on the other.
The tablets for the 15 mg and 30 mg strengths have a breakline and can be broken into two halves if required.
Axit 15, Axit 30 and Axit 45 are available in blister packs of 30 tablets.
Ingredients
The active ingredient in Axit is mirtazapine. Each Axit tablet contains either 15 mg, 30 mg or 45 mg of mirtazapine.
The tablets also contain the following inactive ingredients:
- lactose monohydrate
- maize starch
- colloidal anhydrous silica
- hyprolose
- magnesium stearate
- Opadry Buff OY-LS-37200 (contains iron oxide yellow CI77492 [E172], iron oxide red CI77491 [E172], iron oxide black CI77499 [E172], in 30 mg tablets only)
- Opadry II complete film coating system 39F52901 Yellow (contains quinoline yellow CI47005 and iron oxide yellow CI77492, in 15 mg tablets only)
- Opadry White OY-LS-28908 (in 45 mg tablets only).
Axit tablets contain trace amounts of sulfites.
The tablets are gluten free.
Manufacturer
Axit is made in Australia by:
Alphapharm Pty Ltd
Level 1, 30 The Bond
30 - 34 Hickson Road
Millers Point NSW 2000
www.mylan.com.au
Australian registration number:
Axit 15 - AUST R 97194 (blister pack)
Axit 30 - AUST R 97195 (blister pack)
Axit 45 - AUST R 164493 (blister pack)
This leaflet was prepared in February 2021.
Axit_cmi\Feb21/00
Published by MIMS March 2021





 Some secondary parameter results have been excluded from Table 3. These were number of:
Some secondary parameter results have been excluded from Table 3. These were number of:
 Chemical name: (±)-1,2,3,4,10,14b- hexahydro-2-methyl-pyrazino [2,1-a]pyrido[2,3-c][2]benzazepine.
Chemical name: (±)-1,2,3,4,10,14b- hexahydro-2-methyl-pyrazino [2,1-a]pyrido[2,3-c][2]benzazepine.