What Is In This Leaflet
Read this leaflet carefully before you start to use BETOPTIC S Eye Drops.
This leaflet has been written to answer some common questions about BETOPTIC S Eye Drops. It does not contain all of the available information and does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. In deciding to prescribe BETOPTIC S Eye Drops for you, your doctor has weighed the potential risks and the expected benefits of using this medicine.
The information in this leaflet applies to BETOPTIC S Eye Drops only. This information does not apply to similar products, even if they contain the same ingredients.
If you have any concerns about using BETOPTIC S Eye Drops ask your doctor or pharmacist.
Keep this leaflet with your medicine.
You may need to read it again.
What BETOPTIC S Is Used For
BETOPTIC S Eye Drops contain the active ingredient betaxolol hydrochloride. Betaxolol hydrochloride belongs to a class of medicines known as “beta-adrenergic blocking agents”.
Your doctor has prescribed BETOPTIC S Eye drops for you because the pressure within your eye(s), known as “intraocular pressure”, is higher than normal. This raised pressure may damage your eyesight and lead to a condition known as glaucoma.
BETOPTIC S Eye Drops are used, either alone or in combination with other medicines, to lower the raised pressure within your eye(s). BETOPTIC S Eye Drops do this by reducing the amount of fluid produced within your eye(s).
Before You Use BETOPTIC S
Let your doctor know if any of the following applies to you before you start using BETOPTIC S Eye Drops:
- You are allergic to betaxolol hydrochloride or to any of the other ingredients in BETOPTIC S Eye Drops (these are listed under “Product Description”);
- You are pregnant, or are intending to become pregnant;
- You are breastfeeding;
- You have, or have ever had, any type of problems with your heart;
- You have, or have ever had, any type of respiratory disorder (e.g. wheezing or asthma);
- You are diabetic;
- You have an overactive thyroid gland;
- You suffer from any form of muscle weakness;
- You are taking any other medicines, including medicines that you buy at a pharmacy or health food shop without a doctor’s prescription.
- You are a contact lenses wearer
This is particularly important if you are currently using any other type of anti-glaucoma medication or any other eye drops.
BETOPTIC S Eye Drops are not recommended for use in children.
Do not use BETOPTIC S Eye Drops if:
- The seal around the cap is broken;
- The date (EXP) printed on the label and carton has passed.
Do not put BETOPTIC S Eye Drops into your eye(s) while you are wearing soft contact lenses.
You can put your soft contact lenses into your eyes 15 minutes after you have used BETOPTIC S Eye Drops.
Using BETOPTIC S
It is important that you shake BETOPTIC S Eye Drops well before using them.
The usual dose of BETOPTIC S Eye Drops is one drop in the affected eye(s) two times each day. Your dosing instructions will be printed on the label your pharmacist put on the bottle or carton.
Do not use BETOPTIC S Eye Drops more often than your doctor or pharmacist has told you.
If you have been using any other eye drops for the treatment of raised intraocular pressure or glaucoma it may take several days to change from the old drops to BETOPTIC S Eye Drops. It is important that you carefully follow your doctor’s instructions for the changeover.
After using BETOPTIC S Eye Drops wait at least 5 minutes before putting any other eye drops in your eye(s).
If you are going to have a general anaesthetic your doctor may gradually stop your treatment with BETOPTIC S Eye Drops.
If you are unsure about when, or how, to stop using BETOPTIC S Eye Drops you should talk to your doctor.
How to use BETOPTIC S
Follow these steps to use BETOPTIC S Eye Drops:
- Wash your hands thoroughly.
- Immediately before using a bottle for the first time, break the safety seal around the neck area and throw the loose plastic ring away.
- Shake the bottle well.
- Remove the cap from the bottle.
- Hold the bottle upside down in one hand between your thumb and middle finger (see Diagram 1).
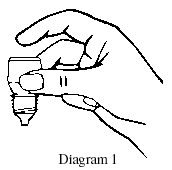
- While tilting your head back, gently pull the lower eyelid of your eye down using the forefinger of your other hand.
- Place the dropper tip close to, but not touching, your lower eyelid and gently tap or press the base of the bottle with your forefinger to release one drop (see Diagrams 2 and 3).
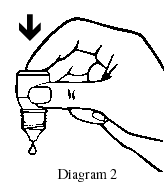
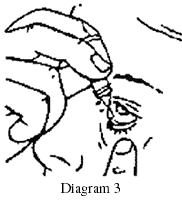
- Close your eye gently without blinking and press on the inside corner of eye with the pad of your index finger for two minutes.
- If necessary repeat the above steps 1-6 for your other eye.
- Place the cap on the bottle and close it tightly.
- Wash your hands again.
You may feel a slight burning sensation in the eye shortly after using BETOPTIC S Eye Drops. If this persists, or is very uncomfortable, contact your doctor or pharmacist.
Do not touch the tip of the dropper to your eye or to any other surface.
This will help to prevent your eye drops becoming dirty or contaminated.
If you forget to use BETOPTIC S
If you forget to use BETOPTIC S Eye Drops you should put the drops that you missed in as soon as you remember and then go back to using them normally. If it is almost time for your next dose, skip the dose that you have missed and take your next dose when you are due to.
Never take a double dose to make up for the one that you missed.
If you are not sure what to do, contact your doctor or pharmacist.
In case of overdose
If you accidentally put too many drops in your eye(s) immediately rinse your eye(s) with warm water or normal saline.
If anyone accidentally swallows BETOPTIC S Eye Drops immediately telephone the nearest Poisons Information Centre (in Australia call 13 11 26; in New Zealand call 0800 POISON or 0800 764 766), your doctor or go to Casualty at the nearest Hospital.
While You Are Using BETOPTIC S
You should:
- Tell your doctor if you become pregnant while you are using BETOPTIC S Eye Drops.
- Tell your doctor and pharmacist that you are using BETOPTIC S Eye Drops before you start taking any other medicines.
Do not:
- Do not let children handle BETOPTIC S Eye Drops. If a child accidentally swallows any of the drops read the instructions under “In case of overdose”.
- Do not stop using BETOPTIC S Eye Drops without first asking your doctor.
- Do not give this medicine to anyone else, even if they appear to have the same condition as you.
You should not drive or operate any machinery if BETOPTIC S Eye Drops affect your vision in any way.
Side Effects
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Tell your doctor as soon as possible if you do not feel well while you are using BETOPTIC S Eye Drops.
Most side effects from BETOPTIC S Eye Drops occur in, or around, the eye. These include:
- Discomfort or pain in the eye(s);
- A loss of feeling to the surface of the eye(s);
- Redness, inflammation, irritation and/or itching in the eye(s), eyelids or the surrounding lining of the eyelids;
- Inflammation of the cornea (clear front portion of your eye) (punctuate keratitis)
- Blurred vision and/or problems seeing clearly;
- A feeling that something is in the eye(s);
- Eyelid spasms
- An increase in tearing or a discharge from the eye(s);
- A dry eye(s);
- Crusty eyelash(es) or eyelids;
- Discomfort in the eye(s) due to a greater sensitivity to light.
- Weakness or easily fatigued eyes
Occasionally some people notice unwanted effects in the rest of their body as a result of using BETOPTIC S Eye Drops. These effects may include:
- Changes in breathing (e.g. wheezing or asthma);
- Cough
- Respiratory infection, sinusitis, runny nose
- Circulation problems;
- Fast or slowing of heart beat, irregular heart beat
- Nausea
- Trouble sleeping;
- Headache;
- Dizziness;
- Fainting
- Tiredness and/or depression;
- Decreased libido
- Hives and more severe forms of skin rash;
- Flaking skin and/or hair loss;
- Sore tongue.
- Altered taste sensation
Let your doctor know if you observe any unwanted effects while using BETOPTIC S Eye Drops, even if they do not appear in the lists above.
After Using BETOPTIC S
Store BETOPTIC S Eye Drops in a cool place, below 25°C. Do not freeze BETOPTIC S Eye Drops. Store the bottle in the outer carton.
Do not leave BETOPTIC S Eye Drops in the car and do not leave them in the bathroom or in other warm, damp places.
Keep BETOPTIC S Eye Drops, and all other medicines, in a safe place away from children.
Discard each bottle of BETOPTIC S Eye Drops 4 weeks after it has been opened. Write the date the bottle was opened on the label to remind you when to discard the bottle.
Product Description
Pack size: 5 mL DROP-TAINER®
Active drug: betaxolol hydrochloride (equiv. to betaxolol 2.5 mg/mL)
Preservative: benzalkonium chloride (0.1 mg/mL)
Other ingredients: polystyrene sulfonate, carbomer 934P, mannitol, disodium edetate, purified water
AUST R: 42900
Further Information
This leaflet does not contain all of the information available about BETOPTIC S Eye Drops. If you have any questions, or are not sure about anything regarding your medicine, you should ask your doctor or pharmacist.
Supplied By
In Australia this product is supplied by:
Alcon Laboratories (Australia) Pty Ltd
25 Frenchs Forest Road East
FRENCHS FOREST NSW 2086
In New Zealand this product is distributed by:
Alcon New Zealand Limited
c/o Pharmaco NZ Limited
4 Fisher Cresent
Mt Wellington, Auckland
This leaflet was prepared on
11 December 2012.
®Registered Trademark


 No evidence of cardiovascular beta-adrenergic blockade during exercise was observed with betaxolol in a double masked crossover study in 24 normal subjects comparing ophthalmic betaxolol hydrochloride solution 1.0% and placebo for effects on blood pressure and heart rate2. Mean arterial blood pressure was not affected by any treatment; however, ophthalmic timolol produced a significant decrease in the mean heart rate (see Table 2).
No evidence of cardiovascular beta-adrenergic blockade during exercise was observed with betaxolol in a double masked crossover study in 24 normal subjects comparing ophthalmic betaxolol hydrochloride solution 1.0% and placebo for effects on blood pressure and heart rate2. Mean arterial blood pressure was not affected by any treatment; however, ophthalmic timolol produced a significant decrease in the mean heart rate (see Table 2).