What is in this leaflet
This leaflet answers some common questions about ENTECAVIR VIATRIS.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have benefits and risks. Your doctor has weighed the risks of you taking ENTECAVIR VIATRIS against the benefits expected for you.
If you have any concerns about taking this medicine, talk to your doctor or pharmacist.
Keep this leaflet with your medicine. You may need to read it again.
What ENTECAVIR VIATRIS is used for
This medicine belongs to a group of medicines called antiviral medicines.
This medicine is used to treat adults infected with hepatitis B virus.
Infection by hepatitis B virus can lead to damage to the liver. This medicine reduces the amount of virus in your body, and has been shown to improve the condition of the liver.
It is not known how safe ENTECAVIR VIATRIS is when taken for long periods.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
This medicine is not addictive.
This medicine is available only with a doctor's prescription.
This medicine is not recommended for use in children under 16 years, as there have been no studies of its effects in children.
Before you take ENTECAVIR VIATRIS
When you must not take it
Do not take ENTECAVIR VIATRIS if you have an allergy to:
- any medicine containing entecavir monohydrate.
- any of the ingredients listed at the end of this leaflet
Some of the symptoms of an allergic reaction may include
- chills fever
- fast heart beat
- wheezing or coughing
- difficulty breathing
- dizziness
- flushing
- sweating and swelling of the face, lips, tongue or other parts of the body
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
It is important to remain under the care of your doctor during ENTECAVIR VIATRIS therapy and after stopping ENTECAVIR VIATRIS. You should report any new symptoms, medication or any other aspects affecting your health to your doctor. Your hepatitis B virus infection may get worse if you stop taking ENTECAVIR VIATRIS. If your doctor advises you to stop ENTECAVIR VIATRIS, they will monitor your health and perform regular blood tests to monitor your liver.
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you are pregnant or plan to become pregnant or are breastfeeding. Experience is limited with the use of ENTECAVIR VIATRIS in pregnant women. Therefore, it should not be used during pregnancy unless it is clearly needed. If there is an urgent need to consider ENTECAVIR VIATRIS during pregnancy, your doctor will discuss with you the risks and benefits involved. If you take ENTECAVIR VIATRIS while you are pregnant, talk to your doctor about how you can take part in the entecavir Pregnancy Registry. The purpose of the pregnancy registry is to collect information about the health of you and your baby.
It is not known whether ENTECAVIR VIATRIS passes into breast milk. Therefore to avoid possible side effects in the nursing infant, mothers should stop breast-feeding if they are taking ENTECAVIR VIATRIS.
Tell your doctor if you have or have had any of the following medical conditions:
- currently experience or have experienced any medical conditions especially any problems with your kidneys.
- have HIV and you are not currently on HIV treatment.
ENTECAVIR VIATRIS is not recommended in patients who have both HIV and Hepatitis B and who are not currently receiving anti-HIV treatment. ENTECAVIR VIATRIS may affect your HIV virus which could impact on future treatment options for HIV. - are lactose intolerant. ENTECAVIR VIATRIS contains lactose.
If you have not told your doctor about any of the above, tell him/her before you start taking ENTECAVIR VIATRIS.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from a pharmacy, supermarket or health food shop. Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
How to take ENTECAVIR VIATRIS
Follow all directions given to you by your doctor and pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the box/bottle, ask your doctor or pharmacist for help.
How much to take
The usual dose of ENTECAVIR VIATRIS is 0.5 mg or 1 mg once a day.
If you have a medical problem with your kidneys your doctor may need to change how often you take your ENTECAVIR VIATRIS tablets.
Your doctor will tell you what dose to take and how often you should take your ENTECAVIR VIATRIS tablets.
How to take it
Swallow the tablets whole with a full glass of water.
The dose of ENTECAVIR VIATRIS should be taken on an empty stomach.
When to take it
Take your medicine any time of day provided it is on an empty stomach. Empty stomach means at least 2 hours before food or 2 hours after food.
Talk to your doctor or pharmacist to work out when it is best for you to take your dose of ENTECAVIR VIATRIS.
How long to take it
Continue taking your medicine for as long as your doctor tells you to. This medicine helps to control your condition, but does not cure it. It is important to keep taking your medicine even if you feel well and for as long as your doctor tells you to.
Your doctor has prescribed ENTECAVIR VIATRIS to prevent hepatitis B virus from further damaging your liver.
ENTECAVIR VIATRIS is very important treatment that can improve the inflammation and scar tissue caused by the Hepatitis B virus in your liver and may reduce the change of developing cirrhosis, liver failure and liver cancer.
It is extremely important that you do not stop taking ENTECAVIR VIATRIS without discussing it with your doctor. If ENTECAVIR VIATRIS is suddenly stopped, the hepatitis B virus can become very active again and lead to sudden development of severe liver failure. There is high risk of dying if liver failure develops and liver transplantation may be necessary to save your life.
It is important to take ENTECAVIR VIATRIS every day or as directed by your doctor, to not miss medicine doses, and to make sure you have enough supply until you next see your doctor.
Do not stop taking ENTECAVIR VIATRIS or change the dose unless asked to do so by your doctor, even if you feel better, as it can be very dangerous.
If you forget to take it
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take it as soon as you remember, and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for the dose you missed. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much ENTECAVIR VIATRIS. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
While you are taking ENTECAVIR VIATRIS
Things you must do
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking ENTECAVIR VIATRIS.
Tell any other doctors, dentists and pharmacists who treat you that you are taking this medicine.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking this medicine. It may affect other medicines used during surgery.
If you become pregnant while taking this medicine, tell your doctor immediately.
If you are about to have any blood tests, tell your doctor that you are taking this medicine. It may interfere with the results of some tests.
Keep all of your doctor's appointments so that your progress can be checked.
Things you must not do
Do not take ENTECAVIR VIATRIS to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop taking your medicine or change the dosage without checking with your doctor. If you stop taking it suddenly, your condition may worsen.
Things to be careful of
Be careful driving or operating machinery until you know how ENTECAVIR VIATRIS affects you. This medicine may cause dizziness, in some people. It is not known if this was caused by ENTECAVIR VIATRIS. If you have this symptom, do not drive, operate machinery or do anything else that could be dangerous.
If you feel light-headed, dizzy or faint when getting out of bed or standing up, get up slowly. Standing up slowly, especially when you get up from bed or chairs, will help your body get used to the change in position and blood pressure. If this problem continues or gets worse, talk to your doctor.
Make sure that you visit your doctor regularly throughout your entire course of treatment with ENTECAVIR VIATRIS.
When your treatment with ENTECAVIR VIATRIS is stopped, your doctor will continue to monitor you and take blood tests for several months.
There is no evidence that ENTECAVIR VIATRIS reduces the risk of infecting other with hepatitis B through sexual contact or body fluids (including blood contamination).
Therefore, it is important to take appropriate precautions to prevent others being infected with hepatitis B.
Talk to your doctor about safe sexual practices that protect your partner. Never share needles. Do not share personal items that can have blood or bodily fluids on them, like toothbrushes and razor blades. A vaccine is available to protect those at risk of becoming infected with hepatitis B.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking ENTECAVIR VIATRIS.
This medicine helps most people with hepatitis B infection, but it may have unwanted side effects in some people.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by the following list of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- diarrhoea
- indigestion
- tiredness
- headache
The above list includes the more common side effects of your medicine.
Tell your doctor as soon as possible if you notice any of the following signs or symptoms of liver problems:
- skin and the white part of eyes turns yellow (jaundice)
- urine turns dark
- bowel movement (stool) turn light in colour
- loss of appetite
- nausea
- lower stomach pain
- lactic acidosis
- serious liver problems
Lactic acidosis is a serious medical emergency that can cause death. Lactic acidosis must be treated in the hospital. Reports of lactic acidosis with ENTECAVIR VIATRIS generally involved patients who were seriously ill due to their liver disease or other medical condition.
The above list includes serious side effects that may require medical attention.
Tell your doctor as soon as possible if you notice any of the following signs or symptoms of lactic acidosis:
- feeling very weak or tired
- unusual muscle pain
- trouble breathing
- stomach pain with nausea and vomiting
- feeling cold (especially in arms and legs)
- feeling dizzy or light-headed
- fast or irregular heartbeat
Some people who have taken medicines like ENTECAVIR VIATRIS have developed serious liver problems called hepatotoxicity, with liver enlargement (hepatomegaly) and fat in the liver (steatosis). Hepatomegaly with steatosis is a serious medical emergency that can cause death.
The above list includes serious side effects that may require medical attention.
If any of the following happen, tell your doctor immediately or go to Accident and Emergency at your nearest hospital if you notice any of the following signs of a sudden life-threatening allergic reaction:
- chills
- fever
- fast heart beat
- wheezing or coughing
- difficulty breathing
- dizziness
- flushing
- sweating and swelling of the face, tongue or other parts of the body.
The above list includes very serious side effects. You may need urgent medical attention or hospitalisation.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Other side effects not listed above may also occur in some people.
After taking ENTECAVIR VIATRIS
Storage
Keep your tablets until it is time to take them. If you take the tablets they may not keep well.
Keep your tablets in a cool dry place where the temperature stays below 25°C.
Do not store ENTECAVIR VIATRIS or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
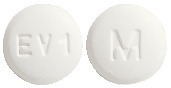
0.5 mg - A white, film-coated, round, biconvex, beveled edge tablet debossed with 'M' on one side of the tablet and 'EV1' on the other side. Blister pack of 30 tablets.
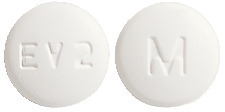
1 mg - A white, film-coated, round, biconvex, beveled edge tablet debossed with 'M' on one side of the tablet and 'EV2' on the other side. Blister pack of 30 tablets.
Ingredients
ENTECAVIR VIATRIS 0.5 mg film coated tablet contains 0.5 mg of entecavir (as monohydrate) as the active ingredient.
ENTECAVIR VIATRIS 1 mg film coated tablet contains 1 mg of entecavir (as monohydrate) as the active ingredient.
It also contains the following inactive ingredients:
- lactose monohydrate
- microcrystalline cellulose
- crospovidone
- magnesium stearate
- Opadry White YS-1R-7003 (ARTG PI No. 1625)
ENTECAVIR VIATRIS contains sugars as lactose.
Supplier
ENTECAVIR VIATRIS is supplied in Australia by:
Alphapharm Pty Ltd trading as Viatris
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.viatris.com.au
Phone: 1800 274 276
This leaflet was prepared in November 2022.
Australian registration numbers:
ENTECAVIR VIATRIS 0.5 mg: AUST R 220090
ENTECAVIR VIATRIS 1 mg: AUST R 220091
ENTECAVIR VIATRIS_cmi\Nov22/00
Published by MIMS December 2022



 Among entecavir treated patients in these studies, on treatment ALT elevations > 10 x ULN and > 2 x baseline generally resolved with continued treatment. A majority of these exacerbations were associated with a > 2 log10/mL reduction in viral load that preceded or coincided with the ALT elevation. Periodic monitoring of hepatic function is recommended during treatment.
Among entecavir treated patients in these studies, on treatment ALT elevations > 10 x ULN and > 2 x baseline generally resolved with continued treatment. A majority of these exacerbations were associated with a > 2 log10/mL reduction in viral load that preceded or coincided with the ALT elevation. Periodic monitoring of hepatic function is recommended during treatment.
 Causes of death in study AI463048 were generally liver related, as expected in this population. The time to onset of HCC or death (whichever occurred first) was comparable in the two treatment groups.
Causes of death in study AI463048 were generally liver related, as expected in this population. The time to onset of HCC or death (whichever occurred first) was comparable in the two treatment groups.



 Responses for patients with baseline Knodell fibrosis scores of 4 (cirrhosis) were comparable to overall responses on all efficacy outcome measures (all patients had compensated liver disease). Histologic improvement was independent of baseline HBV DNA or ALT levels.
Responses for patients with baseline Knodell fibrosis scores of 4 (cirrhosis) were comparable to overall responses on all efficacy outcome measures (all patients had compensated liver disease). Histologic improvement was independent of baseline HBV DNA or ALT levels.
 In study AI463026, responses for patients with baseline Knodell fibrosis scores of 4 (cirrhosis) were comparable to overall responses on all efficacy outcome measures (all patients had compensated liver disease). Histologic Improvement was independent of baseline HBV DNA or ALT levels.
In study AI463026, responses for patients with baseline Knodell fibrosis scores of 4 (cirrhosis) were comparable to overall responses on all efficacy outcome measures (all patients had compensated liver disease). Histologic Improvement was independent of baseline HBV DNA or ALT levels.

 At the end of the open label phase (week 48), the mean change from baseline HBV DNA by PCR for patients originally assigned to entecavir was -4.20 log10 copies/mL (n = 43); 4/51 (8%) patients had HBV DNA < 300 copies/mL by PCR; and 13/35 (37%) patients with abnormal ALT at baseline had ALT normalisation. Entecavir has not been evaluated in HIV/HBV coinfected patients who are not concurrently receiving effective HIV treatment (see Section 4.4 Special Warnings and Precautions for Use, Coinfection with HIV).
At the end of the open label phase (week 48), the mean change from baseline HBV DNA by PCR for patients originally assigned to entecavir was -4.20 log10 copies/mL (n = 43); 4/51 (8%) patients had HBV DNA < 300 copies/mL by PCR; and 13/35 (37%) patients with abnormal ALT at baseline had ALT normalisation. Entecavir has not been evaluated in HIV/HBV coinfected patients who are not concurrently receiving effective HIV treatment (see Section 4.4 Special Warnings and Precautions for Use, Coinfection with HIV). Dosage adjustment is recommended for patients with a creatinine clearance < 50 mL/min, including patients on haemodialysis or CAPD. (See Section 4.2 Dose and Method of Administration, Renal impairment.)
Dosage adjustment is recommended for patients with a creatinine clearance < 50 mL/min, including patients on haemodialysis or CAPD. (See Section 4.2 Dose and Method of Administration, Renal impairment.) Chemical name: 2-amino-1,9-dihydro-9-[(1S,3R,4S) -4-hydroxy-3- (hydroxymethyl)- 2-methylenecyclopentyl]- 6H-purin-6-one, monohydrate.
Chemical name: 2-amino-1,9-dihydro-9-[(1S,3R,4S) -4-hydroxy-3- (hydroxymethyl)- 2-methylenecyclopentyl]- 6H-purin-6-one, monohydrate.