SUMMARY CMI
FAVIC
Consumer Medicine Information (CMI) summary
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
1. Why am I using FAVIC?
FAVIC contains the active ingredient famciclovir. It is an antiviral medicine that is used to treat recurrent outbreaks of cold sores in adults who have a normal immune system.
For more information, see Section 1. Why am I using FAVIC? in the full CMI.
2. What should I know before I use FAVIC?
Do not use if you have ever had an allergic reaction to FAVIC or any of the ingredients listed at the end of the CMI. Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding.
For more information, see Section 2. What should I know before I use FAVIC? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with FAVIC and affect how it works.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
4. How do I use FAVIC?
Your doctor will give you instruction on how to take FAVIC tablets.
More instructions can be found in Section 4. How do I use FAVIC? in the full CMI.
5. What should I know while using FAVIC?
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Drinking alcohol |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using FAVIC? in the full CMI.
6. Are there any side effects?
Side effects of this medicine may include headache; dizziness; abdominal pain or bloating; nausea; vomiting; diarrhoea; itching or an itchy rash (urticaria); abnormal liver function test results; extreme sleepiness; hallucinations; painful or swollen joints; aching muscles; yellowing of the skin or eyes; palpitations; a rash on other parts of your body; swelling below the surface of the skin; severe blistering of the skin; unexplained bruising; purple patches, itching, burning of the skin; seizures or fits; difficulty breathing or swallowing, wheezing or coughing.
For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
FULL CMI
FAVIC Treatment of Cold Sores
Active ingredient(s): famciclovir
Consumer Medicine Information (CMI)
This leaflet provides important information about using FAVIC. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using FAVIC.
Where to find information in this leaflet:
1. Why am I using FAVIC?
2. What should I know before I use FAVIC?
3. What if I am taking other medicines?
4. How do I use FAVIC?
5. What should I know while using FAVIC?
6. Are there any side effects?
7. Product details
1. Why am I using FAVIC?
FAVIC is an antiviral medicine that is used to treat recurrent outbreaks of cold sores in adults who have a normal immune system.
Cold sores are an infection caused by a virus called herpes simplex type 1 (HSV-1). The infection is most commonly acquired as a baby or child from contact with parents or relatives, often from kissing.
Cold sores usually begin on or around the lips, mouth, and nose as small red bumps that turn into fluid-filled blisters. Cold sores can be tender and painful. Many people who get cold sores know when one is coming by a tingling, burning, itchy or painful sensation or redness in the area. This can happen very rapidly.
After redness and swelling develop, blisters form. The blisters may weep or burst and this can be painful. Then a shallow ulcer and yellow crust form as the cold sore dries. The crust eventually falls off, exposing new pink-coloured skin. Generally, the sores heal without scarring. After the initial infection has healed, the virus becomes dormant in nerve cells.
Cold sores can be unpredictable. The virus can become active again in the body, even after many years, resulting in recurrent outbreaks.
Even after many years, some people may experience recurring cold sores due to viral reactivation.
Some common triggers to a cold sore may include:
- sun exposure
- stress
- fatigue
- menstrual periods
- fever
- illness
- dry chapped lips
- skin trauma
- a cold.
FAVIC does not cure the viral infection, however it helps to relieve the symptoms and shorten the duration of an outbreak.
The best results are obtained if the medicine is started as soon as possible after the first symptoms are noticed. These include tingling, itching or burning, or the appearance of redness or swelling. This is when the virus is reproducing rapidly.
2. What should I know before I use FAVIC?
Warnings
Do not use FAVIC if you are allergic medical conditions:
- Famciclovir, the active ingredient
- Penciclovir, a related antiviral medicine
- Any of the ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include shortness of breath, Skin rash, itching or hives, swelling of the face, lips, tongue or other parts of the body, wheezing or troubled breathing.
Do not use FAVIC if:
- the packaging is torn or shows signs of tampering
- the expiry date printed on the pack has passed.
Tell your doctor or pharmacist if you are allergic to any other medicines, foods, preservatives, or dyes.
Tell your doctor if you have or have had any of the following medical conditions:
- problems with your immune system (which helps fight off infections)
- kidney disease
- liver disease.
Your doctor may want to take special care if you have any of these conditions.
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant.
Talk to your doctor if you are breastfeeding or intend to breastfeed.
FAVIC should not be used during pregnancy unless necessary. Your doctor will discuss with you the potential risks of taking FAVIC during pregnancy, and will also advise you if you should take FAVIC while breast-feeding, based on the benefits and risks of your personal situation.
Talk to your doctor or pharmacist if you are not sure whether you should start taking this medicine.
If you have not told them about any of the above, tell him/her before you start taking FAVIC.
All medicines have benefits and risks. Your doctor has weighed the risks of you taking FAVIC against the benefits they expect it will have for you.
There is no evidence that FAVIC is addictive.
FAVIC is not recommended for use in children or adolescents under 18 years of age.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and FAVIC may interfere with each other. These include:
- probenecid, a prescription medicine used to treat gout (a disease with painful, swollen joints caused by uric acid crystals) and to increase blood levels of penicillin-type antibiotics
- raloxifene, a medicine used to treat osteoporosis (a disease which causes bones to become less dense, gradually making them weaker, more brittle and likely to break)
- medicines that can affect your kidneys.
You may need to take different amounts of these medicines or you may need to take different medicines. Your doctor and pharmacist have more information.
Your doctor OR Pharmacist can tell you what to do if you are taking any of these medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
4. How do I use FAVIC?
The usual dose is three 500 mg tablets taken together as a single dose. Swallow the tablets whole with a full glass of water.
The tablets may be taken with or without food. It is not necessary to chew or crush the tablet.
Follow all directions given to you by your doctor or pharmacist carefully.
These directions may differ from the information contained in this leaflet.
If you do not understand the instructions on the box, ask the doctor or pharmacist for help.
When to take FAVIC
Take FAVIC tablets as soon as possible after the first symptoms of a cold sore appear.
Symptoms include tingling, itching or burning or the appearance of redness and swelling.
Do not take the tablets if a hard crust has already formed on the cold sore.
Keep the tablets for the next episode.
How long to take it for
A single dose of FAVIC is all that is necessary for treating each episode of cold sores. The dose may be repeated if cold sores recur. Each pack of FAVIC for cold sores contains enough medicine for one dose.
If you use too much (Overdose)
You should immediately:
- phone the Poisons Information Centre
(by calling 13 11 26) for advice, or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
You may need medical attention.
5. What should I know while using FAVIC?
Things you should do
If you become pregnant, make sure you tell your doctor or pharmacist before taking any further doses of FAVIC.
They can discuss with you the risks of taking it while you are pregnant.
Remind your doctor and pharmacist that you are taking FAVIC if you are about to be started on any new medicine.
Tell any other doctors, dentists and pharmacists who are treating you that you are taking FAVIC.
Things you should not do
Do not take less than the recommended dose of 3 tablets, unless advised by your pharmacist.
If you stop your tablets suddenly, your condition may worsen or you may have unwanted side effects.
Do not take FAVIC to treat any other conditions unless your doctor or pharmacist tells you to.
Do not give FAVIC to anyone else, even if they have the same condition as you.
Driving or using machines
Be careful driving or operating machinery until you know how FAVIC affects you.
This medicine can cause dizziness, sleepiness or confusion in some people.
Things that may help your condition
Cold sores are contagious and the virus can be passed on from person to person through close physical contact or saliva, even when blisters are not present. The risk is much higher when the cold sore is visible, as the virus can be shed, making it easy to infect other people.
Take the following precautions to avoid spreading the virus:
- keep the areas affected by the virus as clean and dry as possible
- avoid touching or scratching the sore area as you may spread the virus on your fingers
- do not share any objects that have been in contact with a cold sore (e.g. drinking glasses, eating utensils, or towels)
- avoid direct skin-to-skin contact of the area with other people (e.g. kissing) until the cold sore has healed.
Looking after your medicine
Keep your tablets in the blister pack, until it is time to take them.
If you take the medicine out of the pack it may not keep well.
Keep your tablets in a cool dry place where the temperature stays below 25°C.
Do not store FAVIC or any other medicines in a bathroom or near a sink.
Do not leave it in the car or on windowsills.
Heat and dampness can destroy some medicines.
Keep FAVIC where children cannot reach it.
A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
When to discard your medicine
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
6. Are there any side effects?
Tell the doctor or pharmacist as soon as possible if you or your child do not feel well while you are taking FAVIC.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by the following lists of side effects.
You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Less serious side effects
| Less Serious Side effects | What to do |
| Speak to your doctor or Pharmacist if you have any of these less side effects and they worry you. |
These side effects are usually mild.
Serious side effects
| Serious side effects | What to do |
| Speak to your doctor or Pharmacist as soon as possible if you have any of these side effects. |
| Call your doctor straight away or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
These are serious side effects. You may need urgent medical attention or hospitalisation. These side effects are rare.
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What FAVIC 500 mg tablet contains
| Active ingredient (Main ingredient) | Famciclovir |
| Other ingredients (Inactive ingredients) |
|
Tablets do not contain gluten, lactose, sucrose, tartrazine or any other azo dyes.
Do not take this medicine if you are allergic to any of these ingredients.
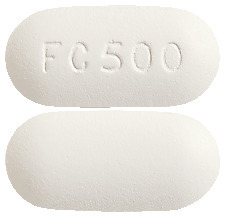
What FAVIC looks like
FAVIC 500 are white to off-white, capsule-shaped tablets with ‘FC 500’ on one.
Each pack contains 30 or 56 tablets.
AUST R 159619
Who distributes FAVIC?
Arrotex Pharmaceuticals Pty Ltd
15 – 17 Chapel Street
Cremorne
Victoria 3121
www.arrotex.com.au
This leaflet was prepared in October 2023
Published by MIMS November 2023




 As these recommendations are not based on repeated dose data, patients with impaired renal function should be closely monitored for adverse effects. There are insufficient data to recommend a dosage for patients with creatinine clearance less than 10 mL/min/1.73 m2.
As these recommendations are not based on repeated dose data, patients with impaired renal function should be closely monitored for adverse effects. There are insufficient data to recommend a dosage for patients with creatinine clearance less than 10 mL/min/1.73 m2. The following adverse events occurred during the clinical trial in recurrent herpes labialis. See Tables 4-6.
The following adverse events occurred during the clinical trial in recurrent herpes labialis. See Tables 4-6.

 Famciclovir has also been well tolerated in immunocompromised patients. Undesirable effects reported from clinical studies were similar to those reported in the immunocompetent population.
Famciclovir has also been well tolerated in immunocompromised patients. Undesirable effects reported from clinical studies were similar to those reported in the immunocompetent population.
