What is in this leaflet
This leaflet answers some common questions about Giotrif.
It does not contain all the available information.
It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking this medicine against the expected benefits.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
This leaflet was last updated on the date at the end of this leaflet. More recent information may be available. The latest Consumer Medicine Information is available from your pharmacist, doctor or from www.medicines.org.au and may contain important information about the medicine and its use of which you should be aware.
Keep this information with the medicine. You may need to read it again.
What Giotrif is used for
Giotrif contains the active substance afatinib (as afatinib dimaleate).
Giotrif belongs to a group of medicines called antineoplastic (or anti-cancer) agents.
It works by blocking the activity of a group of proteins from the ErbB family, which includes a protein called Epidermal Growth Factor Receptor (EGFR). These proteins are known to be involved in the growth and spread of cancer cells. By blocking the activity of these proteins Giotrif stops the cancer cells from growing and multiplying.
Giotrif is used to treat adult patients with a type of lung cancer called non-small cell lung cancer (NSCLC):
- of non-squamous type identified with a change (mutation) in the gene for EGFR. Giotrif can be prescribed to you as your first treatment or if your cancer has progressed after receiving chemotherapy
- of squamous type if your cancer has progressed after receiving chemotherapy.
Ask your doctor if you have any questions about why Giotrif has been prescribed for you. Your doctor may have prescribed Giotrif for another reason.
This medicine is available only with a doctor's prescription.
Before you take Giotrif
When you must not take it
Do not take Giotrif if you have ever had an allergy to:
- afatinib dimaleate (the active ingredient) or
- any of the other ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin.
Do not take this medicine if you are pregnant. It may affect your developing baby if you take it during pregnancy.
If you are a woman who could become pregnant, use adequate contraception during Giotrif treatment and for at least 2 weeks after taking the last dose.
Do not breast-feed if you are taking this medicine. The active ingredient in Giotrif may pass into breast milk and there is a possibility that your baby may be affected.
Do not give this medicine to a child under the age of 18 years. Safety and effectiveness in children younger than 18 years have not been established.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you:
- have any liver problems
- have any kidney problems
- have a history of heart problems
- have a history of lung inflammation (interstitial lung disease)
- have a history of gastrointestinal problems
- are receiving medicines which could increase the risk of developing a hole in the wall of your gut, such as steroids (used to treat inflammation and allergies), NSAIDs (used to relieve pain, swelling and other symptoms of inflammation, including arthritis) or anti-angiogenic agents (used to treat cancer)
- have or have had cancer that has spread to the bowel
- use contact lenses and/or have a history of eye problems such as severe dry eyes, inflammation of the front part of the eye (cornea) or ulcers involving the front part of the eye
- cannot tolerate lactose monohydrate.
If you have not told your doctor about any of the above, tell them before you start taking Giotrif. Your doctor may want to take special precautions if you have any of the above conditions.
It is likely that your doctor will also prescribe an anti-diarrhoeal medicine (e.g. loperamide) for you to take in case you get diarrhoea after starting treatment with Giotrif.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you buy without a prescription from your pharmacy or health food shop.
Some medicines and Giotrif may interfere with each other. These include:
- ritonavir, nelfinavir or saquinavir, medicines used to treat HIV infections
- ciclosporin or tacrolimus, medicines used to suppress the immune system
- ketoconazole or itraconazole, medicines used to treat fungal infections
- erythromycin or rifampicin, medicines used to treat infections
- verapamil, a medicine used to treat high blood pressure and angina
- amiodarone, a medicine used to treat irregular heartbeats
- carbamazepine, phenytoin or phenobarbital, medicines used to treat fits or convulsions
- herbal medicines derived from St John's wort (Hypericum perforatum)
- quinidine, a medicine used to treat irregular heartbeats
- sulfasalazine, a medicine used to treat inflammation
- rosuvastatin, a medicine used for lowering cholesterol.
These medicines may be affected by Giotrif or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
Your doctor and pharmacist will have more information on medicines to be careful with or avoid while taking this medicine.
How to take Giotrif
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the box, ask your doctor or pharmacist for help.
How much to take
The recommended dose is one tablet of Giotrif 40 mg each day.
Your doctor may adjust (increase or decrease) your dose depending on how well you tolerate Giotrif. If you get severe diarrhoea or other intolerable side effects, your doctor may interrupt your treatment with Giotrif and then re-start your treatment at a lower dose.
How to take it
Swallow the tablet whole with a full glass of water. Do not chew or crush the tablets.
For patients with swallowing difficulties the tablet can be dissolved in drinking water (non-carbonated). No other liquids should be used. Follow these instructions carefully:
- Drop the tablet into half a glass of drinking water (non-carbonated) (Do not break or crush the tablet)
- Stir the water occasionally for up to 15 minutes until the tablet is broken up into very small particles
- Drink the liquid straight away
- Rinse the empty glass with half a glass of drinking water and drink it.
When to take it
Take your medicine at about the same time each day. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
Take your medicine on an empty stomach. Do not eat for at least 3 hours before taking your medicine and at least 1 hour after taking your medicine. Food can interfere with the absorption of this medicine.
How long to take it
Continue taking your medicine for as long as your doctor tells you.
If you forget to take it
If it is less than 8 hours before your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take it as soon as you remember, and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for the dose that you missed. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (Australia telephone 13 11 26; New Zealand telephone 0800 764 766) for advice, or go to Emergency at the nearest hospital if you think that you or anyone else may have taken too much Giotrif. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
While you are taking Giotrif
Things you must do
If you are about to be started on any new medicine, tell your doctor or pharmacist that you are taking Giotrif.
Tell any other doctors, dentists and pharmacists who treat you that you are taking this medicine.
If you go into hospital, tell the medical staff that you are taking this medicine.
If you become pregnant while taking this medicine, tell your doctor immediately.
Things you must not do
Do not take Giotrif to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop taking your medicine or lower the dosage without checking with your doctor.
Things to be careful of
Limit your exposure to sunlight while you are taking Giotrif. When you are outdoors, wear a hat, protective clothing and sunscreen. Giotrif may cause your skin to be much more sensitive to sunlight than it normally is. Rash or acne may occur or worsen in areas exposed to the sun.
Be careful driving, operating machinery or doing jobs that require you to be alert until you know how Giotrif affects you. No studies on the effects of Giotrif on the ability to drive and operate machinery have been performed.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Giotrif.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if you notice any of the following and they worry you:
- skin reactions such as acne-like rash, sometimes itchy with dry skin
- mouth sores and inflammation
- problems with your nails
- loss of appetite or weight changes
- bleeding from the nose
- pain, redness swelling or peeling of the skin of your hands and feet
- burning sensations during urination and frequent, urgent need to urinate
- abnormal taste sensations
- stomach pain, indigestion, heartburn
- lip inflammation
- runny nose
- muscle spasms
- fever
- nausea
- vomiting.
Giotrif may be associated with changes in your blood, urine or liver test results. Your doctor may want to perform tests from time to time to check on your progress and detect any unwanted side effects.
Tell your doctor immediately if you notice any of the following:
- diarrhoea (usually occurs within the first 2 to 6 weeks of treatment)
- any signs and symptoms of dehydration such as headache, dizziness, tiredness or decreased urine output
- severe skin reactions such as peeling or blistering of the skin
- severe pain in your stomach area, fever, chills, sickness, vomiting, or abdominal rigidity or bloating, this could be symptoms of a hole in the wall of your gut ('gastrointestinal perforation')
- sudden or worse eye problems such as irritated, red, runny or itchy eyes, blurred vision, swollen or crusty eyelid, or dry eye
- a combination of any of the following: breathlessness, swelling of the feet, ankles, legs or stomach, feeling tired, a feeling like your heart is racing or throbbing.
Diarrhoea is a very common side effect of Giotrif and this is sometimes severe. You may become dehydrated if you experience severe or persistent diarrhoea and this could become serious and life-threatening if untreated.
As soon as you notice any signs of diarrhoea, you should drink plenty of fluids and take the anti-diarrhoeal medicine exactly as your doctor tells you to help treat your diarrhoea.
You must immediately ask your doctor for further advice if your diarrhoea becomes severe (with more than 4 bowel movements each day) or if your diarrhoea is not under control within 48 hours after taking the anti-diarrhoeal medicine.
Tell your doctor immediately or go to Emergency at your nearest hospital if you experience sudden difficulty in breathing or unexplained breathing problems associated with cough or fever. Some patients taking Giotrif have experienced a rare form of lung inflammation called interstitial lung disease which is a serious side effect. This side effect is uncommon. You may need urgent medical attention or hospitalisation.
Tell your doctor immediately or go to Emergency at your nearest hospital if you experience:
- severe upper stomach pain radiating to the back, nausea, vomiting, or fever (which may be symptoms of an inflamed pancreas - pancreatitis)
- severe blisters and bleeding in the lips, eyes, mouth, nose and genitals
- painful red areas of the skin, large blisters and peeling of the skin, accompanied by fever and chills, aching muscles and generally feeling unwell.
These side effects are uncommon. You may need urgent medical attention or hospitalisation.
Tell your doctor or pharmacist if you notice anything else that is making you feel unwell. Other side effects not listed above may also occur in some people.
After taking Giotrif
Storage
Keep your tablets in the blister pack until it is time to take them to protect from moisture and light. If you take the tablets out of the blister pack they may not keep well.
Keep your tablets in a cool dry place where the temperature stays below 30°C.
Do not store Giotrif or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any that is left over.
Product Description
What it looks like
Giotrif is the brand name of your medicine.
Giotrif is available in four strengths of film-coated tablets:
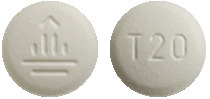
Giotrif 20 mg - white to slightly yellowish, round tablets, imprinted with a "T20" code on one side and the company logo on the other side.
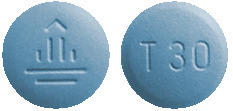
Giotrif 30 mg - dark blue, round tablets, imprinted with a "T30" code on one side and the company logo on the other side.
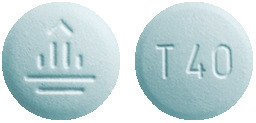
Giotrif 40 mg - light blue, round tablets, imprinted with a "T40" code on one side and the company logo on the other side.
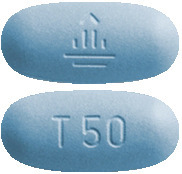
Giotrif 50 mg - dark blue, oval tablets, imprinted with a "T50" code on one side and the company logo on the other side.
Giotrif is packed in blister foils of 7*, 14* and 28 tablets. Each blister foil is packed together with a desiccant sachet in a protective foil pouch.
*Not distributed in Australia.
Ingredients
Active ingredient:
Giotrif 20 mg - 20 mg afatinib (as 29.56 mg afatinib dimaleate) per tablet.
Giotrif 30 mg - 30 mg afatinib (as 44.34 mg afatinib dimaleate) per tablet.
Giotrif 40 mg - 40 mg afatinib (as 59.12 mg afatinib dimaleate) per tablet.
Giotrif 50 mg - 50 mg afatinib (as 73.9 mg afatinib dimaleate) per tablet.
Inactive ingredients:
Each tablet also contains:
- lactose monohydrate
- microcrystalline cellulose
- colloidal anhydrous silica
- crospovidone
- magnesium stearate.
The tablets also have a film-coating which contains:
- hypromellose
- macrogol 400
- titanium dioxide
- purified talc
- polysorbate 80
- colourant containing indigo carmine aluminium lake (only used for 50 mg, 40 mg and 30 mg tablets).
Supplier
Giotrif is supplied in Australia by:
Boehringer Ingelheim Pty Limited
ABN 52 000 452 308
Sydney, Australia
www.boehringer-ingelheim.com.au
Giotrif is supplied in New Zealand by:
Boehringer Ingelheim (N.Z.) Ltd
Auckland
® Giotrif is a registered trade mark of Boehringer Ingelheim.
© Boehringer Ingelheim 2019.
This Consumer Medicine Information was updated in June 2019.
Australian Registration Numbers
GIOTRIF 20 mg AUST R 201314
GIOTRIF 30 mg AUST R 201318
GIOTRIF 40 mg AUST R 201315
GIOTRIF 50 mg AUST R 201320
Published by MIMS August 2019

 Interstitial lung disease (ILD) should be considered if a patient develops acute or worsening of respiratory symptoms in which case Giotrif should be interrupted pending evaluation. If ILD is diagnosed, Giotrif should be discontinued and appropriate treatment instituted as necessary (see Section 4.4 Special Warnings and Precautions for Use).
Interstitial lung disease (ILD) should be considered if a patient develops acute or worsening of respiratory symptoms in which case Giotrif should be interrupted pending evaluation. If ILD is diagnosed, Giotrif should be discontinued and appropriate treatment instituted as necessary (see Section 4.4 Special Warnings and Precautions for Use). In the pivotal LUX-Lung 8 (1200.125) trial a total of 392 patients with Squamous NSCLC were treated with Giotrif with a starting dose of 40 mg once daily and a total of 395 patients were treated with 150 mg erlotinib once daily. After the first treatment cycle (28 days) the dose of Giotrif was escalated to 50 mg in 39 (10%) patients. The overall incidence of ADRs in patients treated with Giotrif or erlotinib was 93% vs. 81% respectively. The incidence of diarrhoea ADRs was higher in the Giotrif-treated patients compared to erlotinib (70% vs. 33%), while incidence of rash/acne was similar in both groups (67% vs. 67%). Dose reductions due to adverse events occurred in 27% of Giotrif-treated patients. Treatment was discontinued due to ADRs in 11% of patients treated with Giotrif, and in 5% of erlotinib treated patients.
In the pivotal LUX-Lung 8 (1200.125) trial a total of 392 patients with Squamous NSCLC were treated with Giotrif with a starting dose of 40 mg once daily and a total of 395 patients were treated with 150 mg erlotinib once daily. After the first treatment cycle (28 days) the dose of Giotrif was escalated to 50 mg in 39 (10%) patients. The overall incidence of ADRs in patients treated with Giotrif or erlotinib was 93% vs. 81% respectively. The incidence of diarrhoea ADRs was higher in the Giotrif-treated patients compared to erlotinib (70% vs. 33%), while incidence of rash/acne was similar in both groups (67% vs. 67%). Dose reductions due to adverse events occurred in 27% of Giotrif-treated patients. Treatment was discontinued due to ADRs in 11% of patients treated with Giotrif, and in 5% of erlotinib treated patients. Liver function test abnormalities (including elevated alanine aminotransferase [ALT] and aspartate aminotransferase [AST]) were observed in patients receiving Giotrif 40 mg. These elevations were mainly transient and did not lead to discontinuation of treatment. Grade 2 (> 2.5 to 5.0 times ULN [upper limit of normal]) ALT elevations occurred in 7.9% and 3.6% of patients treated with Giotrif or chemotherapy, respectively. Grade 3 (> 5.0 to 20.0 times ULN) elevations occurred in 3.5% and 1.8% of patients treated with Giotrif or chemotherapy, respectively (see Section 4.4 Special Warnings and Precautions for Use).
Liver function test abnormalities (including elevated alanine aminotransferase [ALT] and aspartate aminotransferase [AST]) were observed in patients receiving Giotrif 40 mg. These elevations were mainly transient and did not lead to discontinuation of treatment. Grade 2 (> 2.5 to 5.0 times ULN [upper limit of normal]) ALT elevations occurred in 7.9% and 3.6% of patients treated with Giotrif or chemotherapy, respectively. Grade 3 (> 5.0 to 20.0 times ULN) elevations occurred in 3.5% and 1.8% of patients treated with Giotrif or chemotherapy, respectively (see Section 4.4 Special Warnings and Precautions for Use). One patient (0.2%) receiving a 40 mg starting dose experienced grade 4 rash/ acne.
One patient (0.2%) receiving a 40 mg starting dose experienced grade 4 rash/ acne. The safety of Giotrif monotherapy in patients with squamous cell carcinoma of the lung receiving 40 mg starting dose was assessed in trial LUX-Lung 8. The most frequent ADRs were associated with the EGFR inhibitory mode of action of Giotrif and were consistent with trial LUX-Lung 3 in patients with adenocarcinoma of the lung. The majority of patients with ADRs (65%) had Grade 1 or 2 events. The ADR of CTCAE grade 3/4 diarrhoea occurred in 9.9%/0.5% of patients. The rate of drug-related CTCAE grade 3 rash was 5.9%. ADRs led to discontinuation of treatment for 11% of patients.
The safety of Giotrif monotherapy in patients with squamous cell carcinoma of the lung receiving 40 mg starting dose was assessed in trial LUX-Lung 8. The most frequent ADRs were associated with the EGFR inhibitory mode of action of Giotrif and were consistent with trial LUX-Lung 3 in patients with adenocarcinoma of the lung. The majority of patients with ADRs (65%) had Grade 1 or 2 events. The ADR of CTCAE grade 3/4 diarrhoea occurred in 9.9%/0.5% of patients. The rate of drug-related CTCAE grade 3 rash was 5.9%. ADRs led to discontinuation of treatment for 11% of patients.
 Subgroup analyses were conducted based on the stratification factor of EGFR mutation status (Del19, L858R, other) and mutation category (common [Del19, L858R] vs. uncommon [other]). See Figure 2.
Subgroup analyses were conducted based on the stratification factor of EGFR mutation status (Del19, L858R, other) and mutation category (common [Del19, L858R] vs. uncommon [other]). See Figure 2. Of the 26 Giotrif treated patients, eight achieved a partial response (N = 4) or prolonged disease control of longer than 6 months (N = 4): 4 patients with mutations of the category L858R + T790M (1 PR, PFS 11.0 months; 3 SD, 9.6+, 8.3, and 6.7 months); and 1 patient in each with a mutation of the categories L861Q (1 SD, 8.3 months); G719X (1 PR, 10.8 months); S768I + L858R (1 PR, 13.8+ months); and S768I (1 PR, 19.2+ months). The PFS was shorter than 6 months in all patients with the following mutation categories: T790M alone (N = 2), deletion 19 and T790M (N = 3), G719X and T790M (N = 1), exon 20 insertion (N = 6). There were 11 chemotherapy treated patients in the 'other' uncommon EGFR mutation subgroup; of these, four (36%) achieved a partial response.
Of the 26 Giotrif treated patients, eight achieved a partial response (N = 4) or prolonged disease control of longer than 6 months (N = 4): 4 patients with mutations of the category L858R + T790M (1 PR, PFS 11.0 months; 3 SD, 9.6+, 8.3, and 6.7 months); and 1 patient in each with a mutation of the categories L861Q (1 SD, 8.3 months); G719X (1 PR, 10.8 months); S768I + L858R (1 PR, 13.8+ months); and S768I (1 PR, 19.2+ months). The PFS was shorter than 6 months in all patients with the following mutation categories: T790M alone (N = 2), deletion 19 and T790M (N = 3), G719X and T790M (N = 1), exon 20 insertion (N = 6). There were 11 chemotherapy treated patients in the 'other' uncommon EGFR mutation subgroup; of these, four (36%) achieved a partial response. The effect on PFS was consistent across major subgroups, including gender, age, race, ECOG status, and mutation type (L858R, Del 19). The median OS with first-line Giotrif vs. chemotherapy was not significantly different in the common EGFR mutations (Del19 and L858R) subgroup (31.6 months vs. 28.2 months, HR = 0.78, 95% CI [0.58, 1.06], p = 0.1090) or the L858R subgroup (27.6 months vs. 40.3 months, HR = 1.30, 95% CI [0.80, 2.11], p = 0.2919), but was significantly different for the Del19 subgroup (33.3 months vs. 21.1 months, HR = 0.54, 95% CI [0.36, 0.79], p = 0.0015).
The effect on PFS was consistent across major subgroups, including gender, age, race, ECOG status, and mutation type (L858R, Del 19). The median OS with first-line Giotrif vs. chemotherapy was not significantly different in the common EGFR mutations (Del19 and L858R) subgroup (31.6 months vs. 28.2 months, HR = 0.78, 95% CI [0.58, 1.06], p = 0.1090) or the L858R subgroup (27.6 months vs. 40.3 months, HR = 1.30, 95% CI [0.80, 2.11], p = 0.2919), but was significantly different for the Del19 subgroup (33.3 months vs. 21.1 months, HR = 0.54, 95% CI [0.36, 0.79], p = 0.0015).
 Disease-related symptoms were assessed using the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaires (QLQ-C30 and QLQ-LC13). Statistically significant increases in time-to-worsening of pre-specified symptoms 'cough', 'dyspnoea' and 'pain', and improvements in cough, dyspnoea and pain were seen in the afatinib arm, compared to the chemotherapy arm. However, these results may have been affected by the open-label nature of the trial.
Disease-related symptoms were assessed using the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaires (QLQ-C30 and QLQ-LC13). Statistically significant increases in time-to-worsening of pre-specified symptoms 'cough', 'dyspnoea' and 'pain', and improvements in cough, dyspnoea and pain were seen in the afatinib arm, compared to the chemotherapy arm. However, these results may have been affected by the open-label nature of the trial. The PFS hazard ratio for patients with Del 19 mutations and L858R mutations was 0.76 (95% CI [0.55, 0.65]; p = 0.1071), and 0.71 (95% CI [0.47, 1.06]; p = 0.0856) respectively for afatinib vs. gefitinib.
The PFS hazard ratio for patients with Del 19 mutations and L858R mutations was 0.76 (95% CI [0.55, 0.65]; p = 0.1071), and 0.71 (95% CI [0.47, 1.06]; p = 0.0856) respectively for afatinib vs. gefitinib. In patients with tumours harbouring exon 20 insertions (N = 23) the confirmed ORR was 8.7% and the median duration of response was 7.1 months. In patients with tumours harbouring de novo T790M mutations (N = 14) the confirmed ORR was 14.3% and the median duration of response was 8.3 months.
In patients with tumours harbouring exon 20 insertions (N = 23) the confirmed ORR was 8.7% and the median duration of response was 7.1 months. In patients with tumours harbouring de novo T790M mutations (N = 14) the confirmed ORR was 14.3% and the median duration of response was 8.3 months.


