What is in this leaflet
This leaflet answers some of the common questions people ask about Glycoprep-C Kit.
It does not contain all the available information that is known about Glycoprep-C Kit. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor will have weighed the risks of you taking Glycoprep-C Kit against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine.
You may need to read it again.
What Glycoprep-C Kit is used for
Glycoprep-C Kit is used for bowel cleansing in conjunction with intravenous pyelograms (IVP), x-ray examinations, surgery and colonoscopy.
The bowel needs to be clean before your doctor can examine it properly.
Glycoprep-C Kit produces watery stools or bowel motions within 2 to 3 hours after the first administration depending on your individual response.
This medicine belongs to a group of medicines called Bowel Preparations.
Ask your doctor if you have any questions about why this medicine has been prescribed for you.
Before you take Glycoprep-C Kit
When you must not take it
Do not take Glycoprep-C Kit if you have an allergy to:
- Any of the ingredients listed at the end of this leaflet
- Any similar medicines to Glycoprep-C Kit
Some symptoms of an allergic reaction may include:
- Shortness of breath
- Wheezing or difficulty breathing
- Swelling of the face, lips, tongue or other parts of the body
- Skin rash, itching or hives
Do not take Glycoprep-C Kit if you have, or have had, any of the following medical conditions:
- Kidney failure or disease
- Heart failure
- Phenylketonuria - Glycoprep-C contains aspartame
- Gastrointestinal blockage
- Gastric retention - problems with food and fluid emptying from your stomach
- Bowel perforation
- Damage to the entire thickness of the intestinal wall
- Severe distension in the colon
- Blockage in the bowel
- Active inflammatory bowel disease
- A hole in the stomach, large intestine or small intestine
- Toxic megacolon, very dilated large intestine
- Toxic colitis severe inflammation of the large intestine
- A condition known as Paralytic Ileus where the small bowel does not work properly
- Nausea and vomiting
- Severe dehydration
- Appendicitis or inflammation of the appendix
- A body weight less than 20kg.
Ensure you receive adequate fluids during the administration of Glycoprep-C Kit.
Do not take Glycoprep-C Kit after the expiry date printed on the pack, or if the packaging is torn or shows signs of tampering.
If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure when you should start taking Glycoprep-C Kit, talk to your doctor or pharmacist.
Before you start to take it
Tell your doctor or pharmacist if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you have or have had any medical conditions, especially the following:
- Inflammatory bowel disease
- Kidney problems
- Heart problems
- Diabetes
- Dehydration
- Recent gastrointestinal surgery
- Undiagnosed stomach pain
- Ulcerative Colitis
- Stoma - is surgically created when a portion of the small or large intestine is brought out onto the abdomen.
It may not be safe for you to take Glycoprep-C Kit if you have these conditions.
Caution should be exercised in “at risk” patients such as the elderly who are more at risk of dehydration as electrolyte depletion may occur, people with impaired kidney function, diabetes or heart condition. Consult your doctor before use.
Glycoprep-C Kit is not recommended in children as the safety and effectiveness in children has not been established.
Tell your doctor or pharmacist if you are pregnant, intend to become pregnant or are breast-feeding.
Glycoprep-C Kit is not generally recommended for use in pregnant women unless the benefits outweigh the risk to the unborn baby. Your doctor will discuss the benefits and possible risks with you.
Tell your doctor or pharmacist if you are diabetic.
The liquid diet recommended with this medication may affect your blood glucose levels and adjustment of your diabetic medication may be required.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you buy without a prescription from your pharmacy, supermarket or health food shop.
These include:
- Iron medications
- Medicines for heart conditions
- Other bowel cleansing preparations or laxatives
- Medicines used to help the kidneys get rid of salt and water by increasing the amount of urine produced (diuretics)
- Bacitracin and benzylpenicillin.
These medicines may be affected by Glycoprep-C Kit, or may affect how well it works. You may need different amounts of your medicine, or you may need to take different medicines. Your doctor or pharmacist will advise you.
Medications that are taken just before or during the course of Glycoprep-C Kit may not be absorbed. This is due to the increased movement in the digestive tract and the watery diarrhoea that is caused by Glycoprep-C Kit.
- Birth control pills (Oral contraceptives)
- Medicines used to treat infections (antibiotics)
- Medicines for diabetes
Your doctor or pharmacist will have more information on medicines to be careful with or avoid while taking Glycoprep-C Kit.
How to take Glycoprep-C Kit
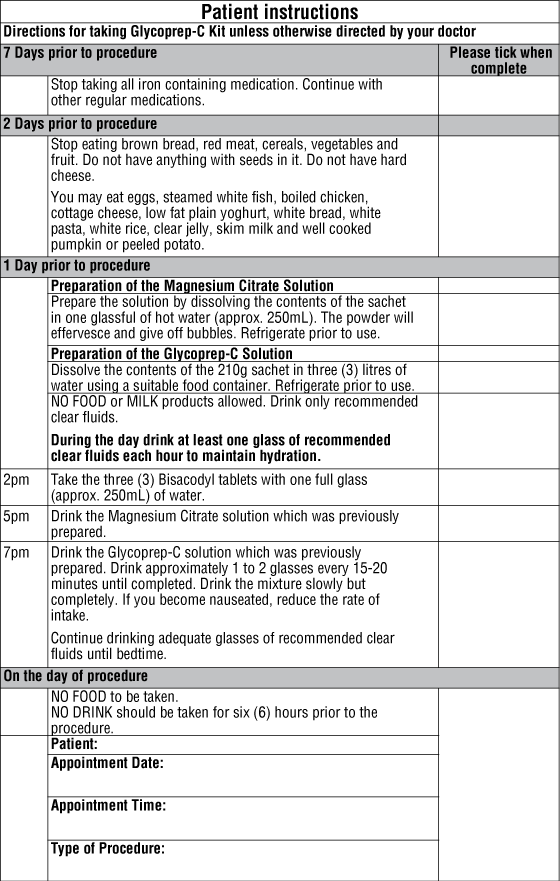
Patient instructions may be found on the carton and in the end of this consumer medicine information leaflet. In some circumstances, your doctor may choose to modify these dose instructions. Follow all directions given to you by your doctor or pharmacist carefully.
They may differ from the information contained in this leaflet.
If you do not understand the instructions on the carton or in this leaflet, ask your doctor or pharmacist for help.
Glycoprep-C Kit will produce numerous bowel motions within a short period of time. Ensure you have bathroom facilities within easy access from when you start to take Glycoprep-C Kit.
The success of your examination depends on the bowel being as clear as possible. Otherwise, the examination may need to be repeated. So it is important to take all the medicine and follow all your doctor's instructions.
How much to take
Glycoprep-C Kit contains only enough medication for one treatment.
The contents may be taken several hours apart on the day before the examination.
How to take it
The glycoprep-C and magnesium citrate sachets must be made up in water before drinking. The detailed instructions for reconstituting the sachets are in the carton.
Swallow the bisacodyl tablets whole.
Do not crush or chew the tablets.
Preparation for the procedure begins seven days prior to the procedure. Refer to the Patient Instructions on the back of this leaflet for details.
Glycoprep-C Kit is taken the day before the procedure unless otherwise stated by your doctor.
It is important that you follow the instructions specifically given by the hospital and that you drink plenty of clear fluids.
Recommended Clear Fluids:
Include water, strained fruit juice without pulp (apple, white grape, pineapple, pear), clear broth, tea or coffee without milk or cream, clear sugar-free cordials such as lemon or lime (no red or purple colourings), plain sugar-free jelly (no red or purple colourings) and clear ice blocks (no red or purple colourings).
Do not drink carbonated beverages.
Do not drink alcoholic beverages.
REMEMBER you need to be close to toilet facilities whilst you are taking Glycoprep-C Kit.
Bowel movements may continue for several hours after the last dose of Glycoprep-C Kit has been taken.
No food to be taken on the day of procedure.
No drink should be taken for six (6) hours prior to the procedure.
If you take too much (overdose)
Overdosage is unlikely as Glycoprep-C Kit contains only enough medication for one treatment.
However in the event of an overdose, dehydration is likely and immediate action should be taken to restore electrolyte balance with appropriate fluid replacement.
Contact the Poisons Information Centre for any further information.
Australia: 13 11 26
New Zealand: 0800 764 766
While you are taking Glycoprep-C Kit
Things you must do
Glycoprep-C Kit can lead to serious dehydration and electrolyte disturbances. You must ensure that you drink the recommended amount of liquid to replace the large amounts of fluid that may be lost during bowel emptying.
Ask your doctor or pharmacist to answer any questions you may have.
Things you must not do
Do not take any additional bowel preparation or laxative products.
Do not give Glycoprep-C Kit to anyone else, even if they have the same condition as you.
Do not stop taking Glycoprep-C Kit or lower the dose without checking with your doctor.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Glycoprep-C Kit.
Glycoprep-C Kit helps most people that require bowel cleansing, but it may have unwanted side effects in a few people. All medicines can have some unwanted side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Ask your doctor or pharmacist to answer any questions you may have.
The following is a list of possible side effects. Tell your doctor or pharmacist if you notice any of the following and they worry you:
- Nausea (feeling sick)
- Vomiting
- Stomach pain
- Stomach bloating
- Anal irritation
- Allergic reaction
Do not be alarmed by this list. You may not experience any of them.
If the effects are severe, you may need medical treatment.
However these side effects usually disappear when treatment with Glycoprep-C Kit is finished.
If you get any side effects, do not stop taking Glycoprep-C Kit without first talking to your doctor or pharmacist.
Other side effects not listed may also occur in some patients. Tell your doctor or pharmacist if you notice anything unusual that is making you feel unwell.
After using Glycoprep-C Kit
Storage
Keep Glycoprep-C Kit in a cool dry place where the temperature stays below 25°C.
Do not store Glycoprep-C Kit or any other medicine in the bathroom or near a sink.
Do not leave it on a window sill or in the car on hot days.
Heat and dampness can destroy some medicines.
Keep it where children cannot reach it.
A locked cupboard at least one and a half metres above the ground is a good place to store medicines.
Disposal
If your doctor or pharmacist tells you to stop taking Glycoprep-C Kit or it has passed the expiry date, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
A carton containing a 210g sachet of white powder labelled Glycoprep-C, a 21.5g sachet of white powder labelled Magnesium Citrate and a blister pack of 3 yellow 5mg Bisacodyl tablets.
Ingredients
Each Glycoprep-C Kit contains the following products:
Each Bisacodyl tablet contains 5mg of bisacodyl as the active ingredient. It also contains:
Lactose, sucrose, gelatin, starch, calcium carbonate, silica-colloidal anhydrous, magnesium stearate, macrogol 6000 and quinoline yellow (104), methacrylic acid copolymer, cellulose microcrystalline, povidone, talc-purified, sodium starch glycolate A and antifoam AF emulsion QF 2587 (PI 1515).
Each sachet labelled Magnesium Citrate contains a white powder for solution, composed of the active ingredients magnesium carbonate - heavy 7.5g and citric acid anhydrous 14g which together forms magnesium citrate in solution.
Each sachet labelled Glycoprep-C contains a white powder for solution composed of macrogol 3350 (polyethylene glycol) 158.7g, sodium chloride 7.8g, potassium chloride 2.2g, sodium sulfate anhydrous 16.9g as the active ingredients. It also contains ascorbic acid, citric acid anhydrous, aspartame and lemon flavour QL58817.
Glycoprep-C Kit does not contain gluten, tartrazine or any other azo dyes.
Glycoprep-C Kit does not contain any preservative.
Australian Registration Number AUST R 20774
Supplier
Fresenius Kabi Australia Pty Limited
964 Pacific Highway
Pymble NSW 2073
Telephone: 1300 361 004
This leaflet was last updated in February 2016.