SUMMARY CMI
RALOVERA®
Consumer Medicine Information (CMI) summary
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
1. Why am I using RALOVERA?
RALOVERA contains the active ingredient medroxyprogesterone acetate. RALOVERA is used to treat endometriosis, the absence of menstrual periods (not due to pregnancy), abnormal bleeding from the uterus, certain types of cancer including cancer of the breast, kidney and endometrium, and in combination with an estrogen containing medicine to relieve symptoms of menopause in women with an intact uterus.
For more information, see Section 1. Why am I using RALOVERA? in the full CMI.
2. What should I know before I use RALOVERA?
Do not use if you have ever had an allergic reaction to medroxyprogesterone acetate or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding.
For more information, see Section 2. What should I know before I use RALOVERA? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with RALOVERA and affect how it works.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
4. How do I use RALOVERA?
- Swallow the tablets whole with a full glass of water at about the same time each day that you need to take it.
More instructions can be found in Section 4. How do I use RALOVERA? in the full CMI.
5. What should I know while using RALOVERA?
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using RALOVERA? in the full CMI.
6. Are there any side effects?
Side effects include: dizziness, increased heart rate, changes to mood or mental state, sleepiness or difficulty sleeping, skin conditions, changes to menstrual period or vaginal secretions, changes to breasts, changes in sex drive, weight and/or appetite changes, fluid retention, yellowing of the skin or eyes, swollen or tender veins, painful swelling in the arms or legs, severe headaches or changes to speech or vision, allergic reactions, chest pain or shortness of breath, hand tremors or cramps.
For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
FULL CMI
RALOVERA®
Active ingredient(s): medroxyprogesterone acetate
Consumer Medicine Information (CMI)
This leaflet provides important information about using RALOVERA. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using RALOVERA.
Where to find information in this leaflet:
1. Why am I using RALOVERA?
2. What should I know before I use RALOVERA?
3. What if I am taking other medicines?
4. How do I use RALOVERA?
5. What should I know while using RALOVERA?
6. Are there any side effects?
7. Product details
1. Why am I using RALOVERA?
RALOVERA contains the active ingredient medroxyprogesterone acetate. RALOVERA is a progestogen, similar to the natural hormone progesterone. Your ovaries produce progesterone during the second half of your monthly menstrual cycle.
RALOVERA is used to treat:
- Endometriosis: a condition in which tissue similar to the lining of the uterus (womb), grows outside the uterus, causing pain and bleeding. RALOVERA helps to stop the growth of this tissue
- Secondary amenorrhoea: a lack of menstrual periods not due to pregnancy. RALOVERA, with or without an estrogen, helps to re-establish a regular menstrual cycle
- Abnormal bleeding from the uterus: when the lining of the uterus breaks down during the menstrual cycle rather than at the end, resulting in vaginal spotting or bleeding. RALOVERA helps to re-establish a regular menstrual cycle
- Certain types of cancer including cancer of the breast, kidney and endometrium
- RALOVERA, in combination with an estrogen containing medicine, is used to relieve symptoms of menopause in women with an intact uterus. This is called hormone replacement therapy (HRT). RALOVERA is used to protect the lining of the uterus while the estrogens relieve the symptoms of menopause. RALOVERA is not suitable as a HRT treatment in women who have undergone a hysterectomy.
Ask your doctor if you have any questions about why RALOVERA has been prescribed for you. Your doctor may have prescribed it for another reason.
2. What should I know before I use RALOVERA?
Warnings
Do not use RALOVERA if:
- you are allergic to medroxyprogesterone acetate, or any of the ingredients listed at the end of this leaflet.
Always check the ingredients to make sure you can use this medicine. - you have or have had any of the following medical conditions:
- a stroke, blood clots including clots in your lungs or anywhere in your body
- severe liver disease
- unusual or irregular vaginal bleeding or blood in your urine that has not been diagnosed by your doctor
- bleeding or discharge from your nipples
- breast cancer, suspected breast cancer or breast lumps not diagnosed by your doctor
- a missed miscarriage
- uncontrolled high blood pressure
- you are pregnant, or suspect you may be pregnant.
- it has passed the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
Check with your doctor if you:
- have allergies to any medicines, foods, preservatives or dyes.
- have or have had any of the following medical conditions:
- heart problems
- kidney problems
- migraine
- brain and/or spinal cord tumour or abnormal growths
- unusual or irregular vaginal bleeding
- genital or breast cancer
- epilepsy
- asthma
- diabetes
- depression
- bone disease or a family history of bone disease, such as brittle bones (osteoporosis)
- vision problems
- fluid retention issues - take any medicines for any other condition.
Some medicines may affect how well RALOVERA works. You may need different amounts of your medicine or you may need to take different medicines. Your doctor will advise you.
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Do not take RALOVERA if you are pregnant or suspect you may be pregnant.
RALOVERA may affect your developing baby if you take it during pregnancy. Check with your doctor if you are pregnant or intend to become pregnant.
Talk to your doctor if you are breastfeeding or intend to breastfeed.
Physical examinations
Before prescribing RALOVERA for you, your doctor may conduct a physical examination which may include breast and pelvic examinations or a mammogram and/or a PAP smear.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Medicines that may reduce the effect of RALOVERA include:
- aminoglutethine, a medicine used for the treatment of breast cancer.
Your doctor or pharmacist may have more information on medicines to be careful with or avoid while taking RALOVERA.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect RALOVERA.
4. How do I use RALOVERA?
How much to take
- Your doctor will tell you how much RALOVERA to take. This will vary depending on the condition for which you are being treated. RALOVERA should be used at the lowest effective dose to treat your condition.
Your doctor may tell you to take RALOVERA every day or in repeating cycles with a break in between. - Follow the instructions provided and use RALOVERA until your doctor tells you to stop.
How to take RALOVERA
- Swallow the tablets whole with a full glass of water.
When to take RALOVERA
- RALOVERA should be taken at about the same time each day. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
How long to take it
- Continue taking your medicine for as long as your doctor tells you.
- Your doctor will prescribe RALOVERA for the shortest duration necessary to effectively treat your condition.
If you forget to take RALOVERA
RALOVERA should be taken regularly at the same time each day.
If you miss your dose at the usual time and it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take it as soon as you remember, and then go back to taking RALOVERA as you would normally.
Do not take a double dose to make up for the dose you missed.
This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much RALOVERA
If you think that you have taken too much RALOVERA, you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre
(by calling 13 11 26), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while using RALOVERA?
Things you should do
- Take RALOVERA exactly as your doctor has prescribed.
- Tell all doctors, dentists and pharmacists who are treating you that you are taking RALOVERA.
- If you are about to start taking any new medicines, tell your doctor and pharmacist that you are taking RALOVERA.
- If you are going to have any laboratory tests, tell your doctor that you are taking RALOVERA. RALOVERA may interfere with the results of some tests.
- Tell your doctor if you feel that RALOVERA is not helping your condition.
- Visit your doctor regularly. Your doctor needs to check your progress and see whether you need to keep taking RALOVERA.
- Regularly check your breasts for any lumps and have regular professional breast examinations and mammograms, as recommended by your doctor.
- Tell your doctor if, for any reason, you have not taken RALOVERA exactly as prescribed.
- Always discuss with your doctor any problems or difficulties during or after taking RALOVERA.
Call your doctor straight away if you:
- become pregnant, or think you may have become pregnant, during treatment. RALOVERA should not be used during pregnancy.
- have sudden partial or complete loss of vision or sudden onset of double vision or migraine.
You will need to be examined and may need to stop taking your medicine.
Things you should not do
- Do not change your dose or stop taking RALOVERA without first checking with your doctor.
- Do not take RALOVERA to treat other complaints unless your doctor tells you to.
- Do not give RALOVERA to anyone else, even if they have the same condition as you.
Bone Mineral Density (BMD) Changes
- The use of RALOVERA may result in a decrease in the amount of calcium in your bones. This could increase your risk of developing brittle bones (osteoporosis), which can lead to bone breakages in later life. This effect can increase with long term use of RALOVERA. The amount of calcium in your bones will start to increase again once you stop treatment with RALOVERA. The time to recovery depends on duration of use. Some women may only partially recover the amount of calcium in their bone.
- If you are taking RALOVERA for prolonged periods, your doctor may also need to evaluate your bone mineral density (BMD).
Ovarian Cancer
- If you use RALOVERA as hormone replacement therapy (HRT) for 5 or more years, your doctor will need to physically check your pelvic organs and conduct blood tests, to rule out the risk of developing ovarian cancer.
Your doctor will discuss the benefits and risks with you and monitor your progress regularly.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how RALOVERA affects you.
RALOVERA may cause dizziness, sleepiness or affect vision in some people.
Drinking alcohol
Tell your doctor if you drink alcohol.
There is no information on how RALOVERA and alcohol may interact.
Looking after your medicine
- Keep RALOVERA in a cool, dry place where the temperature stays below 30°C.
- Keep your tablets in the pack until it is time to take them. If you take the tablets out of the pack they may not keep well.
Follow the instructions in the carton on how to take care of your medicine properly.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on window sills.
Heat and dampness can destroy some medicines.
Do not use this medicine after the expiry date.
Keep it where young children cannot reach it.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
If you are over 65 years of age you may have an increased chance of getting some side effects.
The use of an estrogen at the same time as RALOVERA may also increase the risk of side effects.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Side effects
| Side effects | What to do |
Changes to your mood or energy levels:
| Speak to your doctor if you have any of these side effects and they worry you. |
Feelings of:
Although these side effects are not common, they may require further medical assessment for serious conditions, such as dementia or breast cancer. Soreness, swelling or pain:
The above list includes side effects that may indicate serious conditions that require urgent medical attention or hospitalisation, such as blood clots, heart attack, stroke or severe allergic reactions. Such side effects are not common. | Call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these side effects. |
Some side effects (such as changes in blood pressure) can only be found when your doctor does tests from time to time to check your progress.
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What RALOVERA contains
| Active ingredient (main ingredient) | medroxyprogesterone acetate |
| Other ingredients (inactive ingredients) |
|
Do not take this medicine if you are allergic to any of these ingredients.
What RALOVERA looks like
RALOVERA tablets are available in 5 mg and 10 mg strengths.
RALOVERA 5 mg tablets are pale blue, round and flat, scored and marked "286" on one side and "U" on the other. The 5 mg tablets are available in blister packs of 56 tablets. (AUST R 46531)
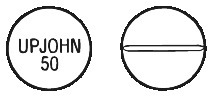
RALOVERA 10 mg tablets are white, round and convex, scored on one side and marked "UPJOHN 50" on the other. The 10 mg tablets are available in blister packs of 30 (AUST R 46532) and bottles of 100 (AUST R 46534).
Who distributes RALOVERA
Pfizer Australia Pty Ltd
Sydney NSW
Toll Free Number: 1800 675 229
www.pfizermedicalinformation.com.au
This leaflet was prepared in March 2024.
Published by MIMS April 2024