SUMMARY CMI
ROXIMYCIN®
Consumer Medicine Information (CMI) summary
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
1. Why am I using ROXIMYCIN?
ROXIMYCIN contains the active ingredient roxithromycin. ROXIMYCIN is used to treat infections in different parts of the body caused by bacteria. For more information, see Section 1. Why am I using ROXIMYCIN? in the full CMI.
2. What should I know before I use ROXIMYCIN?
Do not use if you have ever had an allergic reaction to roxithromycin or any of the ingredients listed at the end of the CMI. Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding. For more information, see Section 2. What should I know before I use ROXIMYCIN? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with ROXIMYCIN and affect how it works. A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
4. How do I use ROXIMYCIN?
- The recommended adult dosage is 300 mg per day.
- The recommended dosage for children more than 40 kg is one 150 mg tablet twice daily.
More instructions can be found in Section 4. How do I use ROXIMYCIN? in the full CMI.
5. What should I know while using ROXIMYCIN?
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Drinking alcohol |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using ROXIMYCIN? in the full CMI.
6. Are there any side effects?
Common side effects include: rash; loss of appetite. For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
FULL CMI
ROXIMYCIN®
Active ingredient: roxithromycin
Consumer Medicine Information (CMI)
This leaflet provides important information about using ROXIMYCIN. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using ROXIMYCIN.
Where to find information in this leaflet:
1. Why am I using ROXIMYCIN?
2. What should I know before I use ROXIMYCIN?
3. What if I am taking other medicines?
4. How do I use ROXIMYCIN?
5. What should I know while using ROXIMYCIN?
6. Are there any side effects?
7. Product details
1. Why am I using ROXIMYCIN?
ROXIMYCIN contains the active ingredient roxithromycin. ROXIMYCIN is an antibiotic that belongs to a group of medicines called macrolides. These antibiotics work by killing or stopping the growth of the bacteria that are causing your infection.
ROXIMYCIN, like other antibiotics, does not work against viral infections such as the flu.
ROXIMYCIN is used to treat infections in different parts of the body caused by bacteria. For example:
- acute pharyngitis (sore throat and discomfort when swallowing)
- tonsillitis
- sinusitis
- acute bronchitis (infection of the bronchi causing coughing)
- exacerbation of chronic bronchitis
- pneumonia (lung infection characterised by fever, malaise, headache)
- skin and soft tissue infections
- non gonococcal urethritis
- impetigo (bacterial infection causing sores on the skin)
2. What should I know before I use ROXIMYCIN?
Warnings
Do not use ROXIMYCIN if:
- you are allergic to roxithromycin, or any other macrolide antibiotic e.g. azithromycin, clarithromycin or erythromycin, or any of the ingredients listed at the end of this leaflet. Symptoms of an allergic reaction may include skin rash, itching, shortness of breath or swelling of the face, lips or tongue which cause difficulty in swallowing or breathing.
Always check the ingredients to make sure you can use this medicine. - you have severe liver problems
- you are taking certain medicines for migraine headache called ergot alkaloids. Ask your doctor if you are not sure if you are taking one of these medicines.
- the product has expired or the packaging appears tampered with.
Check with your doctor if you:
- have any allergies to any other medicines or any other substances, such as foods, preservatives or dyes
- are pregnant or intend to become pregnant
- are breastfeeding or plan to breastfeed
- have or have had the following medical conditions:
- kidney problems (impaired function)
- liver problems (hepatic cirrhosis with jaundice and /or ascites)
- congenital prolongation of the QT interval, with ongoing proarrhythmic heart conditions - have any other medical conditions
- take any medicines for any other condition
- plan to have surgery
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Tell your doctor or pharmacist if you are pregnant or intend to become pregnant. Like most medicines of this kind, ROXIMYCIN is not recommended for use during pregnancy. Your doctor or pharmacist will discuss the risks and benefits of taking it if you are pregnant
Tell your doctor or pharmacist if you are breastfeeding or plan to breastfeed. ROXIMYCIN passes into breast milk. Your doctor or pharmacist will discuss the risks and benefits of taking it if you are breastfeeding or planning to breastfeed.
Use in the Elderly
ROXIMYCIN can be used in the elderly with no dosage adjustment required.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may be affected by ROXIMYCIN, or may affect how well ROXIMYCIN works. These include:
- theophylline (Nuelin), a medicine used to treat asthma
- some medicines for migraine headache such as ergotamine or dihydroergotamine
- disopyramide (Rythmodan), a medicine to treat irregular heart rhythms
- terfenadine and astemizole, over the counter medicines used to treat allergies
- warfarin (Coumadin, Marevan), a medicine used to prevent blood clots
- digoxin (Lanoxin), a medicine used to treat heart failure
- midazolam (Hypnovel), used to induce sleep before operations
- ciclosporin (Neoral, Sandimmun), a medicine used to prevent organ transplant rejection or to treat certain problems with the immune system
- cisapride, a medicine used to treat gastrointestinal problems
- pimozide, an antipsychotic medicine
- hydroxychloroquine or chloroquine, used to treat conditions including rheumatoid arthritis, or to treat or prevent malaria. Taking these medicines at the same time as ROXIMYCIN may increase the chance of you getting side effects that affect your heart.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect ROXIMYCIN.
4. How do I use ROXIMYCIN?
How much to take
- The recommended adult dosage is 300 mg per day which may be taken according to one of the following alternative dosage regimens:
- one 300 mg tablet once a day or
- one 150 mg tablet twice a day or
- two 150 mg tablets once a day
However, depending on your condition and how you react to the medicine, your doctor may ask you to take a different dose. - The recommended dosage for children more than 40 kg is one 150 mg tablet twice daily. The dose for children will depend on the child's weight.
- Your doctor will tell you exactly how much to take.
- Follow the instructions provided and use ROXIMYCIN until your doctor tells you to stop.
When to take ROXIMYCIN
- Take ROXIMYCIN at least 15 minutes before food or on an empty stomach (i.e. more than 3 hours after a meal).
- ROXIMYCIN works best if you take it on an empty stomach. Food in the stomach can reduce the absorption of ROXIMYCIN.
How to take ROXIMYCIN
- Swallow the tablet whole with a glass of water.
How long to take it
- For treating infections, ROXIMYCIN is usually taken for 5 to 10 days. However your doctor may prescribe it for longer periods.
- Continue taking the tablets until you finish the pack or until your doctor or pharmacist tells you to stop
If you forget to use ROXIMYCIN
ROXIMYCIN should be used regularly at the same time each day.
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Do not take a double dose to make up for the dose you missed.
If you use too much ROXIMYCIN
If you think that you have used too much ROXIMYCIN, you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre
(Australia telephone 13 11 26) for advice, or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while using ROXIMYCIN?
Things you should do
- If the symptoms of your infection do not improve within a few days, or if they become worse, tell your doctor.
- If you get severe diarrhoea tell your doctor, pharmacist or nurse immediately. Do this even if it occurs several weeks after ROXIMYCIN has been stopped. Diarrhoea may mean that you have a serious condition affecting your bowel. You may need urgent medical care. Do not take any diarrhoea medicine without first checking with your doctor.
- If you get a sore, white mouth or tongue while taking, or soon after stopping ROXIMYCIN, tell your doctor or pharmacist. Also tell your doctor or pharmacist if you get vaginal itching or discharge.
This may mean you have a fungal/yeast infection called thrush. Sometimes the use of ROXIMYCIN allows fungi/yeast to grow and the above symptoms to occur. ROXIMYCIN does not work against fungi/yeast. - Tell all the doctors, dentists and pharmacists who are treating you that you are taking ROXIMYCIN.
- If you are about to start taking any new medicine, tell your doctor and pharmacist that you are taking ROXIMYCIN.
- If you plan to have surgery that needs a general anaesthetic, tell your doctor or dentist that you are taking this medicine.
Call your doctor straight away if you:
- become pregnant while taking ROXIMYCIN.
Remind any doctor, dentist or pharmacist you visit that you are using ROXIMYCIN.
Things you should not do
- Do not take more than the recommended dose unless your doctor or pharmacist tells you to.
- Do not give this medicine to anyone else, even if they have the same condition as you.
- Do not use this medicine to treat any other complaints unless your doctor tells you to.
- Do not stop taking your tablets or lower the dose because you are feeling better, unless advised by your doctor or pharmacist.
If you do not complete the full course prescribed by your doctor, all the bacteria causing your infection may not be killed. These bacteria may continue to grow and multiply so that your infection may not clear completely or it may return.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how ROXIMYCIN affects you.
ROXIMYCIN may cause dizziness in some people. If you experience any dizziness, do not drive, operate machinery or do anything else that could be dangerous.
Drinking alcohol
Tell your doctor if you drink alcohol.
Looking after your medicine
- Keep your tablets in a cool dry place where the temperature stays below 25°C.
- Keep your tablets in the pack until it is time to take them.
Follow the instructions in the carton on how to take care of your medicine properly.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on window sills.
Keep it where young children cannot reach it.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
| Speak to your doctor if you have any of these less serious side effects and they worry you. These side effects are usually mild and short-lived. |
Serious side effects
| Serious side effects | What to do |
| Tell your doctor or pharmacist immediately if you notice any of these side effects, particularly if they occur several weeks after stopping treatment with ROXIMYCIN. These are rare but serious side effects. You may have a serious condition affecting your bowel. Therefore, you may need urgent medical attention. Do not take any diarrhoea medicine without first checking with your doctor. |
| Stop taking this medicine and call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. These are very serious side effects. If you have them, you may have had a serious allergic reaction to ROXIMYCIN. You may need urgent medical attention or hospitalisation. |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What ROXIMYCIN contains
| Active ingredient (main ingredient) | Each tablet contains either 150 mg or 300 mg of roxithromycin |
| Other ingredients (inactive ingredients) |
|
| Potential allergens | Trace quantities of sulfites |
Do not take this medicine if you are allergic to any of these ingredients.
What ROXIMYCIN looks like
ROXIMYCIN tablets come in 2 strengths:
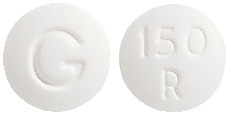
- ROXIMYCIN 150 mg - white, round, film coated, normal convex tablets, marked "150" "R" on one side and "G" on the other. Each blister pack contains 10 tablets (AUST R 99937).
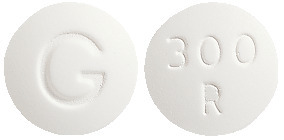
- ROXIMYCIN 300 mg - white, round, film coated, normal convex tablets, marked "300" "R" on one side and "G" on the other. Each blister pack contains 5 tablets (AUST R 99939).
Who distributes ROXIMYCIN
Alphapharm Pty Ltd trading as Viatris
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.viatris.com.au
Phone: 1800 274 276
This leaflet was prepared in October 2023.
ROXIMYCIN® is a Viatris company trade mark
ROXIMYCIN_cmi\Oct23/01
Published by MIMS December 2023

 For atypical pneumonia, the recommended dosage is one 150 mg tablet twice daily.
For atypical pneumonia, the recommended dosage is one 150 mg tablet twice daily. Molecular formula: C41H76N2O15. Molecular weight: 837.07.
Molecular formula: C41H76N2O15. Molecular weight: 837.07.