What is in this leaflet?
This leaflet answers some common questions about Salbutamol AN.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have benefits and risks. Your doctor has weighed the risks of you taking Salbutamol AN against the benefits this medicine is expected to have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
This medicine is only part of a general plan to help you to manage your asthma or other chest conditions. You should discuss this plan with your doctor. Ask your doctor to check your treatment plan regularly.
Keep this leaflet. You may need to read it again.
What Salbutamol AN is used for
The name of your medicine is Salbutamol AN. The medicine in your Salbutamol AN ampoules is delivered through a device called a nebuliser.
Salbutamol belongs to a group of medicines called beta-2-agonists. These work rapidly to open up the air passages in your lungs.
Salbutamol is inhaled into the lungs for the treatment of asthma. Asthma is a disease where the lining of the lungs become inflamed (red and swollen), making it difficult to breathe. This may be due to an allergy to house dust mites, smoke, air-borne pollution and other irritants.
Salbutamol opens up the air passages in people suffering from asthma, bronchitis and other breathing problems. It may also be used before exercise to keep your air passages open if you start to wheeze or have difficulty breathing each time you exert yourself.
Salbutamol may be used for the management of other conditions that are not mentioned above. Your doctor will be able to tell you about the specific condition for which you have been prescribed salbutamol.
This medicine is available only with a doctor's prescription.
Before you use Salbutamol AN
When you must not be given it
Do not use Salbutamol AN if you have an allergy to:
- salbutamol or any other medicines used to treat breathing problems
- any other beta-2-agonist medicine
- any of the ingredients listed at the end of this leaflet
If you are not sure whether any of these apply to you, check with your doctor before you are given it.
Before you are given it:
Tell your doctor if:
- you have any allergies to:
- any other medicines
- any other substances, such as foods, preservatives or dyes
- you are pregnant or intend to become pregnant
- you are breast-feeding or plan to breast feed
- you have or have had any medical conditions, especially the following:
- thyroid problems
- heart problems
- high blood pressure
- diabetes
- glaucoma
Taking other medicines
Tell your doctor if you are taking any other medicines, including any that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and Salbutamol AN may interfere with each other. These include medicines used to treat:
- heart problems
- depression or other mood disorders
- hayfever, coughs and colds
- high blood pressure
- weight reduction
Your doctor will advise you about continuing to take other medicines while you are receiving salbutamol.
How to use Salbutamol AN properly
The contents of Salbutamol AN ampoules are inhaled through a nebuliser according to the manufacturer's instructions. The nebuliser changes the solution into a fine mist and delivers the medicine to your lungs when you inhale the mist through the mask.
The usual dose is 5 mg for an adult and 2.5 mg for a child over four years, given every four to six hours. Your doctor will decide what dose and how often you should use Salbutamol AN. The dosage you will be given will depend on your condition, what it is being used for and other factors, such as your age, and whether or not other medicines are being given at the same time.
Opening instructions for the ampoules:
STEP 1: Remove the strip of Salbutamol AN ampoules from the carton and tear one ampoule from the strip. Open only one foil pack at a time, and use all 5 ampoules before opening the next foil pack.
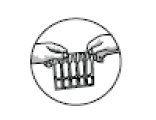
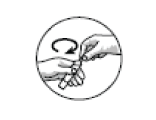
STEP 2: Never use an ampoule that has previously been opened. The ampoule may be opened by carefully holding the ampoule upright and twisting the top off.
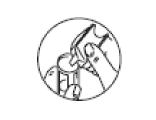
STEP 3: The contents of the ampoule should be squeezed out into the nebuliser bowl.
The nebuliser should be assembled and used as directed by your doctor. After using the nebuliser, discard any solution remaining in the nebuliser bowl. Follow the manufacturer’s instructions on how to clean your nebuliser.
If you forget to take a dose:
If you miss a dose, do not worry. Just take the next dose at the normal time or earlier if you become wheezy or feel tight in your chest.
Important:
Fresh solution must be used for each dose. After the full dose has been given, any solution remaining in the nebuliser must be thrown away. Nebulisers must be cleaned after use according to manufacturer's instructions.
While you are using Salbutamol AN
Things you must do
If you have an Asthma Action Plan that you have agreed with your doctor, follow it closely at all times.
If you find that the usual dose of salbutamol is not giving as much relief as before, or you are needing to use it more often, please contact your doctor so that your condition can be checked.
This is important to ensure that your breathing problem is controlled properly.
Continue using salbutamol for as long as your doctor tells you.
Visit your doctor regularly to check on your condition.
If you become pregnant while using Salbutamol AN tell your doctor.
Tell any other doctors, dentists or pharmacists who are treating you that you are using Salbutamol AN.
Things you must not do
Do not take any other medicines for your breathing problems without checking with your doctor.
Do not give Salbutamol AN to anyone else, even if they have the same condition as you.
Do not use Salbutamol AN to treat any other complaints unless your doctor tells you to.
Side effects
Tell your doctor, nurse or pharmacist as soon as possible if you do not feel well while you are using Salbutamol AN.
Like other medicines, Salbutamol AN can cause some side effects. If they occur, most are likely to be minor or temporary. However, some may be serious and need medical attention.
Ask your doctor or pharmacist to answer any questions that you may have.
Do not be alarmed by this list of possible side effects.
You may not experience any of them.
Tell your doctor immediately if you notice any of the following:
- a feeling of warmth
- difficulty breathing or worsening of your breathing problems
- swelling or severe rash
- fast or irregular heart beat
- pounding heart beat
These may be serious side effects. You may need urgent medical attention. Serious side effects are rare.
Tell your doctor if you notice any of the following and they worry you:
- sore mouth, throat or tongue
- dry mouth
- coughing
- headache
- drowsiness
- feeling anxious, nervous, restless or upset
- difficulty sleeping
- dizziness
- sweating
- trembling or shakiness
- aching or weak muscles
- cramps
- tingling or numbness in the hands or feet ('pins and needles')
- unpleasant taste in your mouth
- nausea or vomiting
- diarrhoea
- rash or itchy skin
- sore or puffy eyes.
These are the more common side effects of salbutamol. Mostly these are mild and short-lived.
Other side effects not listed above may also occur in some patients. If you notice any other effects, check with your doctor. Some side effects may only be seen by your doctor.
If you take too much (overdose)
Immediately telephone your doctor, pharmacist or the Poisons Information Centre (telephone 13 11 26), or go to Accident & Emergency at your nearest hospital, if you think that you or anyone else may have used too much salbutamol. Do this even if there are no signs of discomfort or poisoning. You may need medical attention. If you inhale too much salbutamol, you may experience some of the effects listed under ‘Side Effects’ below.
After you use Salbutamol AN
Storage
Salbutamol AN should be kept in a cool, dry place where the temperature stays below 25°C.
Salbutamol AN must be protected from light.
Do not store in direct sunlight or heat.
Do not leave in the car on hot days.
Keep your Salbutamol AN ampoules in a place where children cannot reach them.
Do not use after the expiry date on the carton.
Once you have opened each foil pack, you need to note down the date of opening the foil lid. Add three to this date and write it down in the space provided on the foil pack.
Do not use the Salbutamol AN ampoules left in the tray after this date.
A locked cupboard at least one and a half metres above the ground is a good place to store medicines.
Do not store Salbutamol AN or any other medicine in the bathroom or near a sink.
Disposal
If your doctor tells you to stop using Salbutamol AN or it has passed its expiry date, ask your pharmacist what to do with any that is left over.
Where to go for further information
Further information can be requested from your doctor or pharmacist.
Product Description
What it looks like
Salbutamol AN contains salbutamol for inhalation. It is a clear, colourless, preservative free solution and comes in sterile plastic unit dose ampoules.
Salbutamol AN comes in two strengths:
- 2.5 mg salbutamol sulfate in 2.5 mL of solution. Available in packs of 30 (6 foil pouches of 5 ampoules) AUST R 135467
- 5 mg salbutamol sulfate in 2.5 mL of solution, Available in packs of 30 (6 foil pouches of 5 ampoules) AUST R 135469
Ingredients
Salbutamol AN contains salbutamol sulfate as the active ingredient.
Salbutamol AN ampoules also contain water for injections and sodium chloride.
Sponsor
Amneal Pharma Australia Pty Ltd
12 River Street
South Yarra VIC 3141
Australia
This Consumer Medicine Information was prepared in Dec 2015.
Salbutamol AN 2.5mg/2.5mL (AUST R 135467)
Salbutamol AN 5mg/2.5mL (AUST R 135469).

