What is in this leaflet
This leaflet answers some common questions about Trileptal.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
The information in this leaflet was last updated on the date listed on the final page. More recent information on the medicine may be available.
You should ensure that you speak to your pharmacist or doctor to obtain the most up to date information on the medicine. You can also download the most up to date leaflet from www.novartis.com.au. Those updates may contain important information about the medicine and its use of which you should be aware.
All medicines have risks and benefits. Your doctor has weighed the risks of you or your child taking this medicine against the benefits they expect it will have.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What Trileptal is used for
Trileptal belongs to a group of medicines called anticonvulsants or antiepileptics. Trileptal is used in adults and children to control some types of epilepsy.
Epilepsy is a condition in which there are repeated convulsions or seizures (fits). Seizures happen because of a temporary fault in the brain's electrical system.
Normally, brain cells coordinate body movements by sending out signals through the nerves to the muscles in an orderly way.
In epilepsy, brain cells send out too many signals in a disorderly fashion. The result can be uncoordinated muscular activity that is called an epileptic seizure.
Trileptal works by keeping the brain's overexcitable nerve cells under control, thereby, reducing the frequency of seizures.
There are two main classes of seizures: generalised and partial.
Generalised seizures -
involve a wide area of the brain, and can affect the whole body.
- Tonic-clonic (or grand mal) seizures are a sub-type of generalised seizure in which the sufferer may lose consciousness and have generalised jerky muscle contractions.
- Absence (or petit mal) seizures are brief, repetitive and involve the whole brain. They usually occur in children and the person loses awareness of what is happening around them. They tend to stare, and roll back their eyes.
Partial seizures -
involve a limited area of the brain (i.e. focal origin), may spread to the whole brain, and may cause a generalised tonic-clonic seizure.
Partial seizures may be simple or complex.
- During simple partial seizures, the patient remains conscious
- In complex partial seizures, the patient's consciousness is altered.
Trileptal may be used alone or in combination with other medicines to treat partial and generalised tonic-clonic seizures in adults and children.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another purpose.
Trileptal is only available with a doctor's prescription.
Trileptal can be used in children aged 1 month and above and in people older than 65 years of age.
There is no evidence that this medicine is addictive.
Before you take Trileptal
When you must not take it
Do not take Trileptal if you have an allergy to:
- oxcarbazepine (the active ingredient in Trileptal)
- eslicarbazepine (another active ingredient related to oxcarbazepine)
- any of the other ingredients of Trileptal listed at the end of this leaflet
Tell your doctor if you are allergic to carbamazepine, the active ingredient in Tegretol® and Teril®, which are other medicines used to treat epilepsy. About 25 to 30% of people who are allergic to carbamazepine are also allergic to oxcarbazepine (Trileptal).
Symptoms of an allergic reaction may include shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue or other parts of the body; rash, itching or hives on the skin.
Tell your doctor if you are of Han Chinese or Thai origin. The risk of serious skin reactions may be predicted by testing your blood. Your doctor should be able to advise if you need a blood test before starting this medicine.
Do not take Trileptal after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. In that case, return it to your pharmacist.
If you are not sure whether you should start taking Trileptal, talk to your doctor or pharmacist.
Before you start to take it
Tell your doctor if you have, or have had, any medical conditions, especially the following:
- liver problems
- kidney problems
- a low level of sodium in your blood
- problems with your heart
Tell your doctor if you have an intolerance to sorbitol, fructose, or parabens. In that case, you should not take Trileptal oral suspension, but you can take Trileptal tablets. Each mL of Trileptal oral suspension contains 175 mg of sorbitol. Sorbitol is converted by the liver to fructose. If people with intolerance to fructose take sorbitol, it can lead to stomach upset and diarrhoea.
Tell your doctor if you are pregnant or intend to become pregnant. It is important to control epileptic seizures during pregnancy. However there may be a risk to your unborn baby if you take Trileptal or other antiepileptic medicines during pregnancy. Your doctor will tell you the benefits and potential risks involved and help you to decide whether you should take Trileptal.
Do not stop your treatment with Trileptal during pregnancy without first checking with your doctor.
Ask your doctor or pharmacist for advice before taking any medicine during pregnancy.
Tell your doctor if you are breastfeeding or plan to breastfeed. The active ingredient in Trileptal passes into breast milk and may possibly affect your baby.
You should not take Trileptal during breast-feeding.
Ask your doctor or pharmacist for advice before taking any medicine during breast-feeding.
Taking other medicines
Tell your doctor if you are taking any other medicines, including medicines that you buy without a prescription from a pharmacy, supermarket or health food shop.
Some medicines and Trileptal may interfere with each other. These include:
- some other medicines used to treat seizures such as phenobarbitone, phenytoin, carbamazepine, rifampicin and valproic acid
- propranolol and calcium antagonists such as felodipine and verapamil, which are medicines used to treat heart problems and high blood pressure
- medicines which reduce the level of sodium in your blood, e.g. diuretic medicines, also called fluid or water tablets, which are used to help the kidneys get rid of salt and water by increasing the amount of urine produced
- diazepam, a medicine used to help you sleep or calm you down
- some medicines used to treat depression, including imipramine, amitriptyline, clomipramine and citalopram
- progesterone, which is often used in hormone replacement therapy (HRT) for menopause and in oral contraceptives (see below)
- cyclophosphamide, a medicine used to treat some types of cancer and to suppress the immune system
- some medicines used to treat stomach ulcers, including omeprazole, lansoprazole and pantoprazole
- St John's wort, which is found in many medicines that you can buy without a prescription in a pharmacy, health food shop or supermarket
- proguanil, a medicine used to treat malaria
- medicines (such as cyclosporin) used to help prevent organ transplant rejection or to treat certain problems with the immune system
The above medicines may be affected by Trileptal or they may affect how well it works. You may need to take different amounts of your medicines or you may need to take different medicines. Your doctor will advise you.
Tell your doctor if you are taking oral contraceptives (birth control pills). If you begin taking Trileptal while you are taking birth control pills, the pill may not work as well as it should. Unplanned pregnancies can happen. Your doctor can suggest another form of birth control while you are taking Trileptal.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking Trileptal.
If you have not told your doctor about any of the above, tell him/her before you take Trileptal.
How to take Trileptal
Follow all directions given to you by your doctor and pharmacist carefully. These directions may differ from the information contained in this leaflet.
If you do not understand the instructions on the label, ask your doctor or pharmacist for help.
How much to take
Before and during your treatment with Trileptal, your doctor may perform blood tests to determine the dose for you. Your doctor will tell you when to have the tests.
Do not exceed the recommended dose.
Adults and Elderly patients:
Your doctor will decide your starting dose. The usual starting dose of Trileptal is 600 mg per day (or 10 mL of the oral suspension).
The dose can be gradually increased if necessary to the amount needed to control your seizures. Maintenance doses are usually between 600 mg and 2400 mg each day (or 10 mL and 40 mL for the oral suspension). Some people will need higher or lower doses than other people.
Patients with kidney disease
The starting dose in impaired renal function is half the usual starting dose.
Children:
Your doctor will calculate the dose, depending on the weight of the child. Your doctor may recommend thyroid function testing before therapy and during therapy (especially in children aged 2 years or below).
How to take it
Take Trileptal twice each day, every day, at about the same time each day, unless your doctor tells you otherwise. Taking your medicine at the same time each day will have the best effect. It will also help you remember when to take it.
Trileptal can be taken either with or without food.
Tablets
Take the tablets with a full glass of water.
If you have to divide any tablets, you can buy a tablet cutter from your pharmacist to make sure the dose is accurate.
Oral suspension:
The bottle is supplied with an oral dosing syringe and a press-in bottle adaptor. Diagrams and instructions on how to use the dispensing system can be found in the carton.
Shake the bottle well and measure the dose immediately afterwards.
The dose that your doctor prescribes you should be given in millilitres (mL) and not in milligrams (mg). This is important because the oral dosing syringe which is used to withdraw the correct dose from the bottle is marked in mL.
If your prescription is in mg, contact your doctor or pharmacist for advice.
Withdraw the prescribed amount of oral suspension from the bottle using the oral dosing syringe supplied.
Swallow the dose directly from the syringe or, if you prefer, mix it in a small glass of water and drink it right away.
Close the bottle and wipe the outside of the oral syringe with a dry, clean tissue.
How long to take it
Continue taking your medicine for as long as your doctor tells you. Trileptal helps to control your epilepsy but does not cure it. You must take this medicine every day, even if you feel well.
Do not stop taking Trileptal or lower the dose without first checking with your doctor. Do not let yourself run out of medicine over the weekend or on holidays. Stopping your medicine suddenly or lowering the dose may cause you to have seizures. Your doctor will usually reduce the dose slowly before you can stop taking it completely.
If you forget to take it
If it is almost time for your next dose of Trileptal, skip the missed dose. Take your next dose at the usual time and continue on with your normal schedule.
If your next dose of Trileptal is not due for quite a while, take a dose as soon as you remember. Then take your next dose at the usual time and continue on with your normal schedule.
Do not take a double dose to make up for the one that you missed. This may increase the chance of you getting an unwanted side effect.
If you are unsure or have forgotten to take several doses, contact your doctor.
If you have trouble remembering when to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or Poisons Information Centre (Phone 13 11 26) for advice or go to Accident and Emergency at your nearest hospital, if you think you or anyone else may have taken too much Trileptal. Do this even if there are no signs of discomfort or poisoning.
Keep the telephone numbers for these places handy.
Some of the symptoms of an overdose may include sleepiness, dizziness, nausea and vomiting, loss of coordination, hyperactivity and rolling of the eyes.
While you are taking Trileptal
Things you must do
Tell your doctor immediately if you become pregnant while taking Trileptal. Your doctor can discuss with you the risks of taking Trileptal while you are pregnant.
Be sure to keep all of your doctor's appointments so that your progress can be checked. This helps to provide you with the best treatment and to prevent unwanted side effects from happening.
Contact your doctor immediately if at any time you have thoughts of harming or killing yourself. A number of people being treated with antiepileptics have had such thoughts or behaviour.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking Trileptal.
Tell any doctor, dentist or pharmacist who treats you that you are taking Trileptal.
Things you must not do
Do not stop taking Trileptal or lower the dose without first checking with your doctor.
Do not use Trileptal to treat any other complaints unless your doctor tells you to.
Do not give this medicine to anyone else, even if their symptoms seem similar to yours or they have the same condition as you do.
Things to be careful of
Ask your doctor if you can drive a vehicle or operate machines. Trileptal may make you feel sleepy or dizzy, cause blurred vision or double vision, and can make you lose muscle coordination.
Be careful driving, operating machinery or doing jobs that require you to be alert, until you know how Trileptal affects you.
There have been reports of bone disorders including osteopenia and osteoporosis (thinning of the bone) and fractures in patients on long term treatment with Trileptal.
Children should avoid doing things like riding bicycles or climbing trees.
This medicine may cause dizziness, drowsiness or blurred vision in some people, especially when you first start to use it or when the dose is increased.
Be careful when drinking alcohol while you are taking Trileptal. Avoid alcohol as much as possible and ask your doctor for advice. Alcohol may increase the sedative effects of this medicine. The combination could make you feel sleepier, dizzier or more light headed than usual.
Side effects
Tell your doctor or pharmacist as soon as possible if you do feel unwell while you are taking Trileptal.
All medicines can have unwanted side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Do not be alarmed by the following list of possible side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- tiredness or drowsiness
- dizziness or spinning sensation
- headache
- weakness, lack of energy
- shakiness, trembling, clumsiness, lack of coordination
- disturbance of balance
- anxiety, agitation, nervousness, depression, mood swings
- apathy
- increase frequency of seizures, especially in children
- difficulty in concentrating, forgetfulness, confusion
- blurred or double vision
- involuntary movements of the eyes
- memory disturbance
- nausea (feeling sick) or vomiting
- constipation
- diarrhoea
- pain in the stomach (abdomen)
- acne
- hair loss
- weight increased
- in very young children (aged 1 month to less than 4 years): lethargy, decreased appetite, irritability, vomiting, rapid uncontrollable movements of the eyes, tremor, clumsiness, painful and swollen joints, and lack of coordination
The above side effects are usually mild. They tend to happen at the start of treatment and usually lessen after a while.
Tell your doctor immediately or go to Accident and Emergency at your nearest hospital if you notice any of the following:
- tiredness, headaches, being short of breath when exercising, dizziness and looking pale
- frequent infections leading to fever, severe chills, sore throat or mouth ulcers
- bleeding or bruising more easily than normal, nose bleeds
- signs of allergy such as swelling of the face, lips, eyelids, throat, mouth, tongue or neck accompanied by difficulty in breathing, speaking or swallowing
- skin rash, or blistering, fever, and pain in the muscle or joints
- severe itching, rash, or hives, fainting, unconsciousness
- signs of a serious skin reaction, such as painful red areas, severe or large blisters, peeling of layers of skin, bleeding in the lips, eyes, mouth, nose, nasal passages or genitals. These signs may be accompanied by fever and chills, aching muscles and feeling generally unwell
- rash, red skin, blistering of the lips, eyes or moth, skin peeling and accompanied by fever, especially in some Asian populations (e.g. Taiwanese, Malaysian, and Philippine) and in patients with Chinese ancestry
- constant "flu-like" symptoms such as chills, fever, sore throat, swollen glands, mouth ulcers, aching joints, lack of energy
- fever with swollen glands
- unusual bleeding or bruising, reddish/purplish patches, or unexplained blotches on the skin
- red blotchy rash mainly on face which may be accompanied by fatigue, fever, nausea, loss of appetite (signs of systemic lupus erythematosus)
- hair loss, weight gain, tiredness, muscle weakness, feeling cold (signs of under active thyroid gland)
- fast, unusually slow, or an irregular heart beat
- persistent nausea, vomiting, severe abdominal pain, loss of appetite, unusual tiredness, yellowing of the skin and/or eyes, dark urine or pale bowel motions (signs of possible pancreatitis or a serious liver problem)
- tiredness, drowsiness, lack of energy, decreased alertness, confusion, nausea, vomiting, blurred vision, muscular twitching, and increased seizures (symptoms of a low level of sodium in the blood)
- flu-like symptoms with yellowing of the skin and/or eyes (signs of hepatitis)
- irregular vaginal bleeding or spotting
- thoughts of harming or killing yourself, as a small number of people being treated with antiepileptics have such thoughts or behaviours
The above signs are serious side effects that may require urgent medical attention.
Tell your doctor if you notice anything else that is making you feel unwell. Some people may have other side effects not yet known or mentioned in this leaflet.
After taking Trileptal
Storage
- Keep your medicine in the original container until it is time to take a dose.
- Store the medicine in a cool dry place where the temperature stays below 30°C.
- Use the oral suspension within 7 weeks after first opening the bottle. After 7 weeks, return any unused oral suspension to your pharmacist for safe disposal.
- Do not store Trileptal or any other medicine in the bathroom or near a sink.
- Do not leave it in the car or on window sills.
Heat and dampness can destroy some medicines.
Keep out of reach of children. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking Trileptal or you find that it has passed the expiry date, ask your pharmacist what to do with any medicine you have left over.
Product description
What it looks like
Trileptal tablets:
Oval tablets marked with a break line on both sides, available in packs of 50 and 100 tablets.
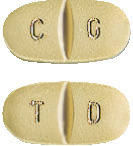
Trileptal 150 mg: pale green tablets coded TD on one side and CG on the other
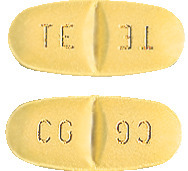
Trileptal 300 mg: yellow tablets coded TE/TE on one side and CG/CG on the other
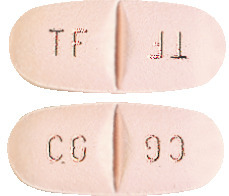
Trileptal 600 mg: light pink tablets coded TF/TF on one side and CG/CG on the other.
Trileptal oral suspension:
An off-white to slightly reddish brown liquid supplied with an oral dosing syringe and a press-in bottle adaptor.
A 10mL oral dosing syringe is supplied with the bottle containing 250mL of Trileptal oral suspension. Discolouration of the oral suspension to a slightly reddish brown colour is normal and does not affect the quality of the medicine.
Ingredients
Trileptal tablets:
Contain 150, 300 or 600 mg of oxcarbazepine as the active ingredient. They also contain:
- silica - colloidal anhydrous
- cellulose - microcrystalline
- hypromellose
- crospovidone
- magnesium stearate
- macrogol 8000 (300 mg tablet only)
- macrogol 4000 (150 and 600 mg tablets only)
- talc - purified
- titanium dioxide
- iron oxide yellow (150 and 300 mg tablets only)
- iron oxide red (150 and 600 mg tablets only)
- iron oxide black (150 and 600 mg tablets only)
Trileptal tablets do not contain lactose, sucrose, gluten, tartrazine or any other azo dyes.
Trileptal oral suspension:
Each mL of oral suspension contains 60 mg of oxcarbazepine. The suspension also contains:
- purified water
- sorbitol (175 mg/mL)
- propylene glycol
- dispersible cellulose
- ascorbic acid (E 300)
- yellow-plum-lemon aroma
- methyl hydroxybenzoate (E 218)
- PEG-8-stearate
- sorbic acid (E 200)
- saccharin sodium
- propyl hydroxybenzoate (E 216).
Trileptal oral suspension contains hydroxybenzoates, saccharin, sorbates and sugar alcohols. Ethanol is a component of the flavour.
Sponsor
Trileptal is supplied in Australia by:
NOVARTIS Pharmaceuticals Australia Pty Limited
ABN 18 004 244 160
54 Waterloo Road
Macquarie Park NSW 2113
Telephone 1-800-671-203
® = Registered trademark
This leaflet was prepared in June 2020.
Australian Registration Number.
Tablets:
Trileptal 150 mg AUST R 76200
Trileptal 300 mg AUST R 76201
Trileptal 600 mg AUST R 76202
Oral suspension
60 mg/mL: AUST R 81195
(tri290620c.doc) based on PI (tri290620i.doc)
Published by MIMS August 2020


 The relative risk for suicidal thoughts or behaviour was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
The relative risk for suicidal thoughts or behaviour was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications. In vivo, plasma levels of phenytoin increased by up to 40% when Trileptal was given at doses above 1200 mg/day. Therefore, when using doses of Trileptal greater than 1200 mg/day during adjunctive therapy, a decrease in the dose of phenytoin may be required (see Section 4.2 Dose and Method of Administration). The increase in the phenobarbitone level, however, is small (15%) when given with Trileptal.
In vivo, plasma levels of phenytoin increased by up to 40% when Trileptal was given at doses above 1200 mg/day. Therefore, when using doses of Trileptal greater than 1200 mg/day during adjunctive therapy, a decrease in the dose of phenytoin may be required (see Section 4.2 Dose and Method of Administration). The increase in the phenobarbitone level, however, is small (15%) when given with Trileptal. Very rarely clinically significant hyponatraemia (sodium < 125 mmol/L) can develop during Trileptal use. It generally occurred during the first 3 months of treatment with Trileptal, although there were patients who first developed a serum sodium < 125 mmol/L more than 1 year after initiation of therapy (see Section 4.4 Special Warnings and Precautions for Use, Hyponatraemia).
Very rarely clinically significant hyponatraemia (sodium < 125 mmol/L) can develop during Trileptal use. It generally occurred during the first 3 months of treatment with Trileptal, although there were patients who first developed a serum sodium < 125 mmol/L more than 1 year after initiation of therapy (see Section 4.4 Special Warnings and Precautions for Use, Hyponatraemia). A monotherapy trial was conducted in 92 paediatric patients (1 month to 16 years of age) with inadequately controlled or new onset partial seizures. Patients were hospitalized and randomized to either Trileptal 10 mg/kg/day or were titrated up to 40-60 mg/kg/day within three days while withdrawing the previous AED on the second day of Trileptal therapy. Seizures were recorded through continuous video EEG monitoring from day 3 to day 5. Patients either completed the 5 day treatment or met one of the two exit criteria 1) three study specific seizures (i.e. electrographic partial seizures with a behavioural correlate), or 2) a prolonged study specific seizure. The primary measure of effectiveness was a between group comparison of the time to meet exit criteria in which the difference between the curves was not statistically significant (p = 0.904). The majority of patients from both dose groups completed the 5-day study without exiting. The percentage of patients exiting the study was 22.2% (10/45) for the low dose group and 21.4% (9/42) for the high dose group.
A monotherapy trial was conducted in 92 paediatric patients (1 month to 16 years of age) with inadequately controlled or new onset partial seizures. Patients were hospitalized and randomized to either Trileptal 10 mg/kg/day or were titrated up to 40-60 mg/kg/day within three days while withdrawing the previous AED on the second day of Trileptal therapy. Seizures were recorded through continuous video EEG monitoring from day 3 to day 5. Patients either completed the 5 day treatment or met one of the two exit criteria 1) three study specific seizures (i.e. electrographic partial seizures with a behavioural correlate), or 2) a prolonged study specific seizure. The primary measure of effectiveness was a between group comparison of the time to meet exit criteria in which the difference between the curves was not statistically significant (p = 0.904). The majority of patients from both dose groups completed the 5-day study without exiting. The percentage of patients exiting the study was 22.2% (10/45) for the low dose group and 21.4% (9/42) for the high dose group. Trial 025 was a placebo controlled trial conducted in 67 untreated patients (8-69 years of age) with newly diagnosed and recent onset partial seizures. Patients were randomized to placebo or Trileptal, initiated at 300 mg BID and titrated to 1200 mg/day (given as 600 mg BID) in 6 days, followed by maintenance treatment for 84 days. The primary measure of effectiveness was a between group comparison of the time to first seizure. The difference between the two treatments was statistically significant in favour of Trileptal (see Figure 2), p = 0.046.
Trial 025 was a placebo controlled trial conducted in 67 untreated patients (8-69 years of age) with newly diagnosed and recent onset partial seizures. Patients were randomized to placebo or Trileptal, initiated at 300 mg BID and titrated to 1200 mg/day (given as 600 mg BID) in 6 days, followed by maintenance treatment for 84 days. The primary measure of effectiveness was a between group comparison of the time to first seizure. The difference between the two treatments was statistically significant in favour of Trileptal (see Figure 2), p = 0.046.
 Trial 028 was a monotherapy substitution trial conducted in 87 patients (11-66 years of age) whose seizures were inadequately controlled on 1 or 2 AEDs. Patients were randomised to either Trileptal 2400 mg/day or 300 mg/day and their standard AED regimen(s) were eliminated over the first 6 weeks of double blind therapy. Double blind treatment continued for another 84 days (total double blind treatment of 126 days) or until one of the 4 exit criteria described for the previous study occurred. The primary measure of effectiveness was a between group comparison of the percentage of patients meeting exit criteria. The results were statistically significant in favour of the Trileptal 2400 mg/day group (14/34; 41.2%) compared to the Trileptal 300 mg/day group (42/45; 93.3%) (p < 0.0001). The time to meeting one of the exit criteria was also statistically significant in favour of the Trileptal 2400 mg/day group (see Figure 4), p = 0.0001.
Trial 028 was a monotherapy substitution trial conducted in 87 patients (11-66 years of age) whose seizures were inadequately controlled on 1 or 2 AEDs. Patients were randomised to either Trileptal 2400 mg/day or 300 mg/day and their standard AED regimen(s) were eliminated over the first 6 weeks of double blind therapy. Double blind treatment continued for another 84 days (total double blind treatment of 126 days) or until one of the 4 exit criteria described for the previous study occurred. The primary measure of effectiveness was a between group comparison of the percentage of patients meeting exit criteria. The results were statistically significant in favour of the Trileptal 2400 mg/day group (14/34; 41.2%) compared to the Trileptal 300 mg/day group (42/45; 93.3%) (p < 0.0001). The time to meeting one of the exit criteria was also statistically significant in favour of the Trileptal 2400 mg/day group (see Figure 4), p = 0.0001.
 Subset analyses of the antiepileptic efficacy of Trileptal with regard to gender in these trials revealed no important differences in response between men and women. Because there were very few patients over the age of 65 in controlled trials, the effect of the drug in the elderly has not been adequately assessed.
Subset analyses of the antiepileptic efficacy of Trileptal with regard to gender in these trials revealed no important differences in response between men and women. Because there were very few patients over the age of 65 in controlled trials, the effect of the drug in the elderly has not been adequately assessed.

 Oxcarbazepine is a white to faintly orange powder; slightly soluble in chloroform, dichloromethane, acetone and methanol; practically insoluble in ethanol, ether and water; sensitive to light in solutions. Melting point: 219°C with decomposition.
Oxcarbazepine is a white to faintly orange powder; slightly soluble in chloroform, dichloromethane, acetone and methanol; practically insoluble in ethanol, ether and water; sensitive to light in solutions. Melting point: 219°C with decomposition.