WHAT IS IN THIS LEAFLET
This leaflet answers some common questions about Zonegran hard capsules.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking this medicine against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist. Keep this leaflet with the medicine. You may need to read it again.
WHAT ZONEGRAN IS USED FOR
The name of your medicine is Zonegran. The active ingredient in Zonegran is called zonisamide.
Zonegran is used to treat certain types of epilepsy in adults. Zonegran may be used:
- On its own to treat seizures in adults.
- With other antiepileptic medicines to treat seizures in adults.
Epilepsy is caused by a disruption in the electrical activity of the brain. The abnormal electrical impulses occur due to altered levels of some chemicals in the brain. Zonegran can control brain chemicals which send signals to nerves so that seizures do not happen.
Zonegran is used in partial seizures that affect only one part of the brain, with or without generalized seizures that may affect the whole brain.
Your doctor may have prescribed Zonegran for another use.
Ask your doctor if you have any questions about why this medicine has been prescribed for you.
There is no evidence that Zonegran is addictive.
This medicine is only available with a doctor’s prescription.
Zonegran capsules is not recommended for children and adolescents under 18 years of age as there is not enough information on its use in children and adolescents.
BEFORE YOU TAKE ZONEGRAN
When you must not take it
Do not take this medicine if you have an allergy to:
- Zonisamide
- Sulphonamide drugs including furosemide (Lasix) and bumetanide (Burinex) (diuretic medicines)
- Any of the ingredients listed at the end of this leaflet (See “Ingredients”)
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin
Do not give this medicines to anyone who is unconscious or in a coma.
Do not give this medicine to anyone under the age of 18 years. Safety and effectiveness in children younger than 18 years have not been established.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If the medicine has expired or the packing is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
You must tell your doctor if:
- You have had an allergic reaction to similar medicines (ask your doctor to check your records).
- You are allergic to any other substances such as food preservatives or dyes
(Zonegran 100mg capsules contain a yellow colour called sunset yellow FCF (E110) which may cause allergic reactions). - You are pregnant or intend to become pregnant.
Like most antiepileptic medicines, Zonegran is not recommended for use during pregnancy. Your doctor will discuss with you the possible risks and benefits of using Zonegran capsules during pregnancy. - You are breast feeding, or wish to breastfeed.
It is recommended that you do not breastfeed while taking or for one month after stopping Zonegran capsules. This medicine may pass into the breast milk and there is a possibility that your baby may be affected. Your doctor will discuss with you the possible risks and benefits of using Zonegran capsules during breastfeeding. - You have liver or kidney disease or kidney stones.
- You have a reduced white blood cell count or have recently lost weight (or weigh less than 40 kg).
- You have high blood levels of ammonia
If you have not told your doctor about any of the above, tell them before you start taking Zonegran.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may affect the way other medicines work. They may also react with Zonegran capsules resulting in untoward or sometimes dangerous side effects. Your doctor or pharmacist will be able to tell you which medicines are safe to take with Zonegran capsules.
Some other antiepileptic drugs such as phenytoin, carbamazepine and phenobarbitone may affect Zonegran, so tell your doctor if you are taking these drugs.
Your doctor or pharmacist will have more information on medicines that you need to be careful with or need to avoid while taking Zonegran capsules.
Before you start to take any other medicines, tell your doctor or pharmacist that you are taking Zonegran capsules.
HOW TO TAKE ZONEGRAN
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions, ask your doctor or pharmacist for help.
How much to take
When you take Zonegran on its own:
The usual starting dose is 100 mg daily taken once a day. This may be increased by up to 100 mg at intervals of two weeks. The usual daily dose is 300 mg once a day.
When you take Zonegran with other antiepileptic medicines:
The usual starting dose is 50mg daily taken in two divided doses. The dose will be gradually increased at intervals of one or two weeks, to a daily dose of between 300mg and 500mg per day. Some patients respond to lower doses.
If you have a kidney problem your doctor may start you on a lower dose and increase it more gradually to prevent unwanted side effects.
Ask your doctor or pharmacist if you are unsure of the correct dose for you. They will tell you exactly how much to take.
Follow the instructions they give you. If you take the wrong dose, Zonegran may not work as well.
How to take it
Zonegran capsules may need to be taken once or twice a day.
Zonegran capsules must be swallowed whole with water. Do not chew the capsules.
Zonegran can be taken with or without food.
When to take Zonegran
Take your medicine at about the same time each day. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
How long to take Zonegran
Continue taking your medicine for as long as your doctor tells you. Do not stop unless your doctor advises you to.
Zonegran capsules help to control your condition, but does not cure it. Therefore, it is important that you keep taking Zonegran capsules every day. Your doctor will check your progress to make sure the medicine is working and will discuss with you how long your treatment should continue.
Zonegran is meant to be taken as a long-term medicine. Do not reduce your dose or stop your medicine unless your doctor tells you to.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
If you forget to take it
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take it as soon as you remember, and then go back to taking your medicine as you would normally.
Do not try and make up for the dose that you missed by taking more than one dose at a time. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone Australia 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much Zonegran. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
WHILE YOU ARE TAKING ZONEGRAN
Things you must do
Tell your doctor if, for any reason, you have not taken your medicine exactly as directed. Otherwise, your doctor may think that it was not working as it should and change your treatment unnecessarily.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking Zonegran.
Tell any other doctors, dentists and pharmacists who treat you that you are taking Zonegran.
If you become pregnant while taking Zonegran, tell your doctor immediately. Do not stop treatment without first discussing it with your doctor. You must only take zonisamide during your pregnancy if your doctor tells you to. If you are a woman of childbearing age you must use adequate contraception while taking zonisamide and for one month after stopping zonisamide.
Your doctor will check your progress and may want to take some blood tests from time to time. This helps to prevent unwanted side effects.
Tell your doctor immediately if you have thoughts about killing yourself or if you are close to or care for someone using Zonegran capsules who talks about or shows signs of wanting to kill him or herself. Persons taking Zonegran capsules may be more likely to think about killing themselves or actually trying to do so, especially when Zonegran capsules are first started or the dose is changed.
If you or someone you know demonstrates any of the following warning signs of suicide-related behaviour while taking Zonegran capsules, contact a healthcare provider immediately, or even go to the nearest hospital for treatment:
- Thoughts or talk of death or suicide
- Thoughts or talk of self-harm or harm to others
- Any recent attempts of self-harm
- New or worse depression
- New or worse anxiety
- Panic attacks
- Trouble sleeping (insomnia)
- Increase in aggressive behaviour, irritability or agitation
- Acting on dangerous impulses
- Extreme increase in activity and talking (mania)
- Other unusual changes in behaviour or mood.
Keep all of your doctor’s appointments so that your progress can be checked.
Things you must not do
Do not use Zonegran to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if their symptoms seem similar to yours.
Do not stop taking your medicine or lower the dosage, even if you are feeling better, without checking with your doctor. If you stop taking Zonegran capsules suddenly, your condition may worsen or your chance of getting an unwanted side effect may increase. To prevent this, your doctor may gradually reduce the amount of Zonegran capsules you take each day before stopping the medicine completely.
Things to be careful of
Do not drive or operating machinery until you know how Zonegran affects you. As with other antiepileptic medicines, Zonegran capsules may cause drowsiness in some people. Make sure you know how you react to Zonegran capsules before you drive a car, operate machinery, or do anything else that could be dangerous.
Make sure you keep cool in hot weather and drink plenty of water. This will help reduce the risk of kidney stones.
SIDE EFFECTS
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Zonegran All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Contact your doctor immediately if you:
- have difficulty breathing, a swollen face, lips or tongue, or a severe skin rash as these symptoms may indicate that you are having a severe allergic reaction.
- have signs of overheating - high body temperature but little or no sweating, rapid heartbeat and breathing, muscle cramps, and confusion.
- have thoughts of harming or killing yourself. A small number of people being treated with antiepileptics such as Zonegran have had thoughts of harming or killing themselves.
- have pain in your muscles or a feeling of weakness, as this may be a sign of abnormal muscle breakdown which can lead to kidney problems.
- get a sudden pain in your back or stomach, have pain on urinating (passing water) or notice blood in your urine, as this may be a sign of kidney stones.
- develop visual problems such as eye pain or blurred vision while taking Zonegran.
Contact your doctor as soon as possible if you:
- have an unexplained skin rash, as this could develop into a more severe skin rash or skin peeling.
- feel unusually tired or feverish, have a sore throat, swollen glands, or find that you bruise more
- easily, as this may mean you have a blood disorder.
- have signs of increased acid level in the blood- headaches, drowsiness, shortness of breath
- have signs of high blood ammonia levels. High ammonia in the blood can affect your mental activities, slow your alertness,
- make you feel tired, or cause vomiting, and
- loss of appetite. Your doctor may need to monitor or treat this.
Your doctor may decide that you should stop using Zonegran
The most common side effects of Zonegran are mild. They occur during the first month of treatment and usually decrease with continued treatment.
Very common side effects (may affect more than 1 in 10 people) are:
- agitation, irritability, confusion, depression
- poor muscle coordination, dizziness, poor memory, sleepiness, double vision
- loss of appetite, decreased blood levels of bicarbonate (a substance that prevents your blood from becoming acidic)
Common side effects (may affect up to 1 in 10 people) are:
- difficulty sleeping, strange or unusual thoughts, feeling anxious or emotional.
- slowed thoughts, loss of concentration, speech abnormalities, abnormal skin sensation (pins and needles), tremor, involuntary movement of the eyes.
- kidney stones.
- skin rashes, itching, allergic reactions, fever, tiredness, flu-like symptoms, hair loss.
- ecchymosis (a small bruise caused by blood leaking from broken blood vessels in the skin).
- loss of weight, nausea, indigestion, stomach pains, diarrhoea (loose stools), constipation.
- swelling of the feet and legs.
Uncommon side effects (may affect up to 1 in 100 people) are:
- anger, aggression, thoughts of suicide, suicide attempt.
- vomiting.
- gall bladder inflammation, gallstones.
- urinary stones.
- lung infection / inflammation, urinary tract infections.
- low blood potassium levels, convulsions/seizures.
Very rare side effects (may affect up to 1 in 10,000 people) are:
- hallucinations, memory loss, coma, neuroleptic malignant syndrome (inability to move, sweating, fever, incontinence), status epilepticus (prolonged or repeated seizures).
- breathing disorders, shortness of breath, inflammation of the lungs.
- inflammations of the pancreas (severe pain in the stomach or back)
- liver problems, kidney failure, increased blood levels of creatinine (a waste product that your kidneys should normally remove).
- severe rashes or skin peeling (at the same time you may feel unwell or develop a fever).
- abnormal muscle breakdown (you may feel pain or weakness in your muscles) which can lead to kidney problems.
- swollen glands, blood disorders (reduction in the number of blood cells, which can make infection more likely and can make you look pale, feel tired and feverish, and bruise more easily).
- decreased sweating, overheating.
- glaucoma, which is a blockage of fluid in the eye causing increased pressure in the eye. Eye pain, blurred vision or decreased vision may occur and can be signs of glaucoma.
- high blood ammonia levels which can affect your mental activities, slow your alertness, make you feel tired, or cause vomiting.
During your treatment with Zonegran capsules, your doctor may from time to time test your blood to look for any side effects which the medication could cause. Your doctor or pharmacist will have more information. Your doctor or pharmacist should discuss the possible side effects of Zonegran capsules with you.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist.
This includes any side effects not listed in this leaflet.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice anything else that is making you feel unwell. Other side effects not listed above may also occur in some people.
AFTER TAKING ZONEGRAN
Storage
Keep your medicine in the original container.
If you take it out of its original container it may not keep well.
Keep your medicine in a cool dry place away from light and where the temperature stays below 25°C.
Do not store Zonegran or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
PRODUCT DESCRIPTION
What it looks like
Zonegran capsules 25mg: a white opaque body and a white opaque cap and printed with “ZONEGRAN 25” in black on the capsule body. Capsule size No 4 containing white powder free from visible impurities.
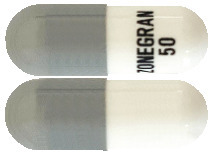
Zonegran capsules 50mg: a white opaque body and a grey opaque cap and printed with “ZONEGRAN 50” in black on the capsule body. Capsule Size No 3 containing white powder free from visible impurities.
Zonegran capsules 100mg: a white opaque body and a red opaque cap and printed with “ZONEGRAN 100” in black on the capsule body. Capsule size No 1 containing white powder free from visible impurities.
Ingredients
Zonegran capsules 25mg
Active Ingredients: Zonisamide
Inactive Ingredients:
- microcrystalline cellulose
- hydrogenated vegetable oil
- sodium laurylsulphate
Capsule shell:
- gelatin
- titanium dioxide (E171)
- TekPrint SW-9008 Black Ink (ARTG No 2328)
Zonegran capsules 50mg
Active Ingredients: Zonisamide
Inactive Ingredients:
- microcrystalline cellulose
- hydrogenated vegetable oil
- sodium laurylsulphate
Capsule shell:
- gelatin
- titanium dioxide (E171)
- black iron oxide (E172)
- TekPrint SW-9008 Black Ink (ARTG No 2328)
Zonegran capsules 100mg
Active Ingredients: Zonisamide
Inactive Ingredients:
- microcrystalline cellulose
- hydrogenated vegetable oil
- sodium laurylsulphate
Capsule shell:
- gelatin
- titanium dioxide (E171)
- allura red AC (E129)
- sunset yellow FCF (E110)
- TekPrint SW-9008 Black Ink (ARTG No 2328)
This medicine does not contain gluten, tartrazine or any other azo dyes.
SPONSOR
Amdipharm Mercury (Australia) Pty Ltd
Level 9, 76 Berry Street
North Sydney NSW 2060
Ph: 1800 627 680
This leaflet was prepared in 13 July 2022
Australian Registration Number
25mg capsules: AUST R 125869
50mg capsules: AUST R 125870
100mg capsules: AUST R 125871
Published by MIMS September 2022




 The relative risk for suicidal thoughts or behaviour was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
The relative risk for suicidal thoughts or behaviour was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
 Other adverse reactions associated with zonisamide obtained from clinical studies and post-marketing surveillance of Zonegran treated patients is included in Table 5.
Other adverse reactions associated with zonisamide obtained from clinical studies and post-marketing surveillance of Zonegran treated patients is included in Table 5.




