What is in this leaflet
Please read this leaflet carefully before you start ABACAVIR/LAMIVUDINE VIATRIS tablets.
This leaflet answers some common questions about ABACAVIR/LAMIVUDINE VIATRIS tablets. It does not contain all of the available information.
It does not take the place of talking to your doctor or pharmacist (also known as a chemist).
All medicines have benefits and risks. Your doctor has weighed the expected benefits of you taking ABACAVIR/LAMIVUDINE VIATRIS tablets against the risks this medicine could have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the tablets. You may need to read it again.
What ABACAVIR/ LAMIVUDINE VIATRIS tablets are used for
ABACAVIR/LAMIVUDINE VIATRIS tablets contain abacavir and lamivudine which belong to a group of medicines called antiretrovirals.
These are also available as separate medicinal products.
ABACAVIR/LAMIVUDINE VIATRIS tablets are used together with other antiretrovirals to slow down the progression of human immunodeficiency virus (HIV) infection, which can lead to Acquired Immune Deficiency Syndrome (AIDS) and other related illnesses (e.g. AIDS-related Complex or ARC).
ABACAVIR/LAMIVUDINE VIATRIS tablets do not cure AIDS or kill the HIV virus, but delays further damage to the immune system by stopping production of new viruses.
You can still pass on HIV when taking this medicine, although the risk is lowered by effective antiretroviral therapy. You will still be able to pass on the HIV virus by sexual activity or by contamination with infected blood. You should still use proper precautions to prevent this.
While taking ABACAVIR/LAMIVUDINE VIATRIS tablets and/or any other therapy for HIV disease, you may continue to develop other infections and other complications of HIV infection. You should keep in regular contact with the doctor who is looking after you.
ABACAVIR/LAMIVUDINE VIATRIS tablets are not addictive.
Before you take ABACAVIR/ LAMIVUDINE VIATRIS tablets
Do not take if:
You must not take ABACAVIR/LAMIVUDINE VIATRIS tablets if:
- you have ever had an allergic reaction to abacavir
- you are allergic to the active ingredient lamivudine or any of the other ingredients listed toward the end of this leaflet.
- you have a serious liver disease ABACAVIR/LAMIVUDINE VIATRIS may not be suitable for you.
Special Warning
ABACAVIR/LAMIVUDINE VIATRIS contains abacavir. Abacavir can cause a serious allergic reaction known as a hypersensitivity reaction, which can be life-threatening if treatment with abacavir containing products is not stopped.
Research has found that people with a gene called HLA-B (type 5701) are more likely to have a hypersensitivity reaction to abacavir. However, even if you do not have this gene type it is still possible for you to get this reaction. If you know you have this gene type, be sure to tell your doctor before you take abacavir.
The most common symptoms of this reaction include high temperature (fever) and a skin rash. Other most frequently seen symptoms include nausea, vomiting, diarrhoea or abdominal pain; severe tiredness or body aches or generally feeling ill; headache; shortness of breath, sore throat or cough. If you develop any of these symptoms call your doctor IMMEDIATELY WHO WILL ADVISE YOU WHETHER YOU SHOULD STOP TAKING ABACAVIR/LAMIVUDINE VIATRIS tablets. If your doctor is not available you must urgently seek other medical advice (e.g. the Accident and Emergency unit of the nearest hospital) before taking the next dose.
Other symptoms may include joint or muscle pain, swelling of the neck or itchy skin. Occasionally inflammation of the eye (conjunctivitis), ulcers in the mouth, tingling or numbness of the hands or feet or low blood pressure may occur. The symptoms of this allergic reaction can occur at any time during treatment with ABACAVIR/LAMIVUDINE VIATRIS tablets. However they usually occur in the first six weeks of treatment, and get worse with continued treatment.
If you have had this serious reaction to ABACAVIR/LAMIVUDINE VIATRIS tablets, NEVER take ABACAVIR/LAMIVUDINE VIATRIS or any other medicinal product containing abacavir again as within hours you may experience a life-threatening lowering of your blood pressure or death.
Occasionally life threatening hypersensitivity reactions have occurred when ABACAVIR/LAMIVUDINE VIATRIS tablets was restarted in patients who reported only one of the symptoms on the Alert Card before stopping.
On very rare occasions, hypersensitivity has been reported when ABACAVIR/LAMIVUDINE VIATRIS tablets were restarted in patients who had no symptoms of hypersensitivity before stopping.
If you have stopped taking ABACAVIR/LAMIVUDINE VIATRIS tablets for any reason it is important that you contact your doctor before restarting. This is especially so if you think you are having side-effects from other medicines or have another illness. Your doctor will check whether any symptoms you had before stopping may be related to this hypersensitivity reaction. If your doctor thinks there is a possibility that they were related, you may be told never to take ABACAVIR/LAMIVUDINE VIATRIS tablets again. It is important that you follow this advice.
If you are hypersensitive to ABACAVIR/LAMIVUDINE VIATRIS tablets you should return all of your unused ABACAVIR/LAMIVUDINE VIATRIS tablets to your doctor or pharmacist for proper disposal.
You must not take ABACAVIR/LAMIVUDINE VIATRIS tablets if:
- you had a previous allergic reaction to ABACAVIR/LAMIVUDINE VIATRIS tablets or other abacavir containing products
- you are pregnant, think you may be pregnant or are breastfeeding, unless your doctor tells you to
- the expiry date (EXP.) printed on the pack has passed
- the packaging is torn or shows signs of tampering.
You must tell your doctor if:
- you are allergic to foods, dyes, preservatives or any other medicines
- you have any other illness
- you are taking any other medicines, including medicines you buy without a prescription from a supermarket or health food shop.
Abacavir or lamivudine in ABACAVIR/LAMIVUDINE VIATRIS tablets may interact with certain other medicines. ABACAVIR/LAMIVUDINE VIATRIS tablets should not be taken with emtricitabine.
Some medicines may affect how ABACAVIR/LAMIVUDINE VIATRIS works, or make it more likely that you'll have side effects. These medicines include:
- sorbitol-containing medicines (usually liquids) used regularly
- trimethoprim-sulphamethoxazole (also known as co-trimoxazole), (an antibiotic used to treat PCP Pneumocystis jiroveci pneumonia (often referred to as PCP) or toxoplasmosis).
Tell your doctor or pharmacist if you are taking any of these.
If you are taking methadone, your doctor may need to adjust your methadone dose, as abacavir increases the rate at which methadone leaves your body. This is unlikely to affect most methadone users.
How do I take ABACAVIR/ LAMIVUDINE VIATRIS tablets?
How much to take
Take ABACAVIR/LAMIVUDINE VIATRIS tablets as directed by your doctor or pharmacist. The normal dose for adults and adolescents is one tablet once a day. Your doctor may prescribe a different dosage.
How to take them
ABACAVIR/LAMIVUDINE VIATRIS tablets should be swallowed whole with water. ABACAVIR/LAMIVUDINE VIATRIS tablets do not have to be taken with food.
How long to take them for
Because your medicine helps to control your condition, but does not cure it, you will need to take the tablets every day. Do not stop taking ABACAVIR/LAMIVUDINE VIATRIS tablets or change the dose without first talking to your doctor.
What do I do if I take too much? (Overdose)
If you think you or anyone else may have taken too many ABACAVIR/LAMIVUDINE VIATRIS tablets, immediately telephone your doctor or Poisons Information Centre (In Australia telephone 131126. In New Zealand telephone 0800 POISON). Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
If you are not sure what to do, contact your doctor or pharmacist.
While you are taking ABACAVIR/ LAMIVUDINE VIATRIS tablets
Things you must do
Tell your doctor if, for any reason, you have not taken your medicine exactly as directed. Otherwise, your doctor may think that it was not effective and change your treatment unnecessarily.
If you forget to take them
It is important to take this medicine as prescribed to ensure you get maximum benefit. If you forget to take a dose, take it as soon as you remember, and then continue as before.
Do not take more than one tablet to make up for the doses you have missed.
If you have stopped taking them
If you have stopped taking ABACAVIR/LAMIVUDINE VIATRIS tablets for any reason, it is important that you contact your doctor before restarting. This is especially so if you think you are having side-effects or have another illness. In some cases your doctor will ask you to restart ABACAVIR/LAMIVUDINE VIATRIS tablets where medical care can be readily accessed by yourself or others.
Things you must not do
Do not give this medicine to anyone else, even if their symptoms seem similar to yours.
Do not use ABACAVIR/LAMIVUDINE VIATRIS tablets to treat any other complaints unless your doctor tells you to.
If you have hepatitis B infection, you should not stop ABACAVIR/LAMIVUDINE VIATRIS tablets without instructions from your doctor, as you may have a recurrence of your hepatitis. This may occur due to you suddenly stopping lamivudine.
Things to be careful of
Be careful driving or operating machinery until you know how ABACAVIR/LAMIVUDINE VIATRIS tablets affect you.
No studies on the effects of ABACAVIR/LAMIVUDINE VIATRIS tablets on the ability to drive and use machines have been performed. However, you should take into account the state of your health and the possible side effects of ABACAVIR/LAMIVUDINE VIATRIS tablets before considering driving or using machines.
What are the side-effects?
Check with your doctor as soon as possible if you think you are experiencing any side-effects or allergic reactions while taking ABACAVIR/LAMIVUDINE VIATRIS tablets, even if the problem is not listed in this leaflet.
Like all medicines, ABACAVIR/LAMIVUDINE VIATRIS tablets can cause some side-effects. If they occur, they are most likely to be minor and temporary. However, some may be serious and may need medical attention.
Hypersensitivity Reaction
ABACAVIR/LAMIVUDINE VIATRIS contains abacavir.
Abacavir can cause a serious allergic reaction known as a hypersensitivity reaction, which can be life-threatening if treatment with abacavir containing products is not stopped. This is described in the section "Special warning" under "Before you take ABACAVIR/LAMIVUDINE VIATRIS tablets", of this leaflet.
It is important that you read and understand the information about this serious reaction.
As ABACAVIR/LAMIVUDINE VIATRIS tablets contains both abacavir and lamivudine, the side effects reported for each of these have been combined.
The most common side-effects (could affect at least one to ten in every 100 people) are:
- nausea, vomiting
- diarrhoea
- upper abdominal pain
- headache
- high temperature
- lethargy, fatigue, loss of appetite
- hair loss
- joint and muscle pain
- abacavir hypersensitivity
- skin rash (without any other illness).
Uncommon side-effects (could affect less than one in every 100 people) are:
- increases in enzymes produced by the liver
- anaemia (low red blood cell count)
- neutropenia (low white blood cell count)
- reduction in the number of platelets (blood cells important for blood clotting).
If the production of red blood cells is reduced, you may have symptoms of tiredness or breathlessness.
A reduction in your white blood cell count can make you more prone to infection. If you have a low platelet count, you may notice that you bruise more easily.
Rare side-effects (could affect less than one in every 1,000 people) are:
- breakdown of muscle tissue, increases of an enzyme called amylase
- inflammation of the pancreas (pancreatitis).
Very rare side-effects (could affect less than one in every 10,000 people) are:
- serious skin reactions
- severe anaemia.
Changes in the amounts of fatty substances and glucose in the blood have also been reported. Within the first few weeks of treatment with anti-HIV medicines, some people, particularly those that have been HIV positive for some time, may develop inflammatory reactions (e.g. pain, redness, swelling, high temperature) which may resemble an infection and may be severe. It is thought that these reactions are caused by a recovery in the body's ability to fight infections, previously suppressed by HIV. If you become concerned about any new symptoms, or any changes in your health after starting HIV treatment, please discuss with your doctor immediately.
In babies and infants exposed to Nucleoside Reverse Transcriptase Inhibitors (NRTIs) during pregnancy or labour small temporary increases in blood levels of a substance called lactate have been observed. Additionally there have been very rare reports of diseases that affect the nervous system such as a delayed development and seizures. Overall, in children whose mothers took NRTIs during pregnancy, the benefit from the reduced chance of being infected with HIV is likely to be greater than the risk of suffering from side effects.
Call your doctor IMMEDIATELY if you notice any of the following. The doctor will tell you whether you should stop taking ABACAVIR/LAMIVUDINE VIATRIS tablets and what you should do:
- Lactic Acidosis
Some people taking ABACAVIR/LAMIVUDINE VIATRIS, or other medicines like it (NRTIs), develop a condition called lactic acidosis, together with an enlarged liver.
Lactic acidosis is caused by a build-up of lactic acid in the body. It is rare; if it happens, it usually develops after a few months of treatment. It can be life-threatening, causing failure of internal organs.
Lactic acidosis is more likely to develop in people who have liver disease, especially in women.
Signs of lactic acidosis include:
- deep, rapid, difficult breathing
- drowsiness
- numbness or weakness in the limbs
- feeling sick (nausea), being sick (vomiting)
- stomach pain. - Allergic (anaphylactic) reaction
The symptoms of an allergic (anaphylactic) reaction which may occur soon after starting ABACAVIR/LAMIVUDINE VIATRIS include wheezing, swelling of the lips/mouth, difficulty in breathing, hayfever, lumpy rash (hives) or fainting. - Old infections may flare up
People with advanced HIV infection (AIDS) have weak immune systems, and are more likely to develop serious infections (opportunistic infections). When these people start treatment, they may find that old, hidden infections flare up, causing signs and symptoms of inflammation. These symptoms are probably caused by the body's immune system becoming stronger, so that the body starts to fight these infections.
Fat gain and loss
Fat gain and loss has been observed with combined antiretroviral therapy. A causal relationship for this has not been established. Should any change in body shape be noticed, seek medical advice.
This is not a complete list of all possible side-effects. Others may occur in some people and there may be some side-effects not yet known.
Do not be alarmed by this list of possible side-effects. You may not experience any of them.
How do I store ABACAVIR/ LAMIVUDINE VIATRIS tablets?
Keep this medicine where children cannot reach it, such as a locked cupboard.
Keep ABACAVIR/LAMIVUDINE VIATRIS tablets in a cool, dry place where it stays below 25°C.
Do not store the tablets, or any other medicine, in a bathroom or near a sink.
Do not leave them in the car or on window sills.
Keep your ABACAVIR/LAMIVUDINE VIATRIS tablets in their pack until you take them.
If your doctor tells you to stop taking ABACAVIR/LAMIVUDINE VIATRIS tablets, or the tablets have passed their expiry date, return any unused or expired medicine to your pharmacist.
Product description
What ABACAVIR/LAMIVUDINE VIATRIS tablets look like
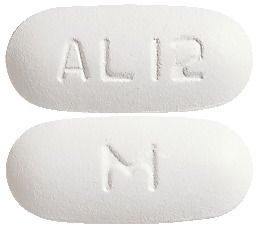
The tablets are white, film-coated, modified capsule shaped, biconvex tablet debossed with AL12 on one side of the tablet and M on the other side.
ABACAVIR/LAMIVUDINE VIATRIS tablets are supplied in blister packs containing 30 tablets.
Ingredients
ABACAVIR/LAMIVUDINE VIATRIS tablets contain 600 mg of abacavir as the sulfate salt and 300 mg of lamivudine.
The tablets also contain the following inactive ingredients:
- microcrystalline cellulose
- crospovidone
- magnesium stearate
- silicified microcrystalline cellulose
- colloidal
- anhydrous silica
- talc
- tablet coating OPADRY II complete film coating system 13B58894 White (ARTG PI No. 13119) containing: hypromellose, titanium dioxide, macrogol 400 and polysorbate 80.
ABACAVIR/LAMIVUDINE VIATRIS tablets contain sorbates and sulfites.
Supplier
ABACAVIR/LAMIVUDINE VIATRIS tablets are supplied by:
Alphapharm Pty Ltd trading as Viatris
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.viatris.com.au
Phone: 1800 274 276
Australian registration numbers:
ABACAVIR/LAMIVUDINE VIATRIS 600 mg/300 mg tablet blister pack: AUST R 266516
Where to go for further information
Pharmaceutical companies are not in a position to give people an individual diagnosis or medical advice. Your doctor or pharmacist is the best person to give you the individual advice you need. You may also be able to find out more information about your disease from books, for example in public libraries.
Counselling is also available from your local AIDS council.
This leaflet was prepared in July 2022.
ABACAVIR/LAMIVUDINE VIATRIS_cmi\Jul22/00
Published by MIMS September 2022

 See Table 2.
See Table 2.

 The abacavir once daily group was demonstrated to be non-inferior when compared to the twice daily group in the overall and base-line viral load sub-groups. The incidence of adverse events reported were similar in the two treatment groups.
The abacavir once daily group was demonstrated to be non-inferior when compared to the twice daily group in the overall and base-line viral load sub-groups. The incidence of adverse events reported were similar in the two treatment groups. Molecular formula: (C14H18N6O)2.H2SO4.
Molecular formula: (C14H18N6O)2.H2SO4. Molecular formula: C8H11N3O3S.
Molecular formula: C8H11N3O3S.