1 Name of Medicine
Aciclovir.
2 Qualitative and Quantitative Composition
Each tablet contains 200 mg or 800 mg aciclovir.
For the full list of excipients, see Section 6.1 List of Excipients.
3 Pharmaceutical Form
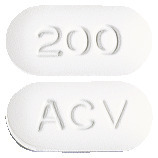
200 mg.
Capsule shaped, biconvex, white to off-white tablets debossed "200" on one side and "ACV" on the other side.
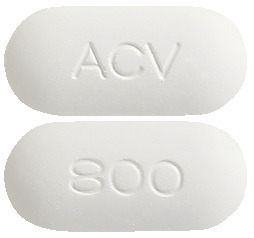
800 mg.
Capsule shaped, biconvex, white to off-white tablets debossed "800" on one side and "ACV" on the other side.4.1 Therapeutic Indications
Adults.
Treatment of first episode (primary or nonprimary) genital herpes and the management of recurrent episodes of genital herpes in certain patients.
Treatment of acute attacks of herpes zoster (shingles) when the duration of rash is less than 72 hours.
The management of patients with advanced symptomatic human immunodeficiency virus (HIV) disease (CD4+ counts < 150 x 106/L).
Genital herpes.
Initial episodes. The duration of viral shedding is reduced very significantly; the duration of pain and time to healing are also reduced. The promptness of initiation of therapy and/or the patient's prior exposure to herpes simplex virus (HSV) may influence the degree of benefit from therapy.
Intravenous aciclovir should be considered in patients in whom prostration, CNS involvement or inability to take oral medication requires hospitalisation and initiation of more aggressive management.
Aciclovir does not prevent the establishment of latency in initial episodes.
Recurrent episodes. Suppression.
In patients with frequent recurrences, suppressive therapy prevents or reduces the frequency and/or severity of recurrences in a high proportion of patients. Abortive episodes (prodromal symptoms without vesicle formation) and occasional breakthrough episodes may, however, continue to occur during suppressive therapy.
Suppressive therapy is not considered appropriate for patients in whom attacks are mild, last for short periods and/or occur infrequently (e.g. less frequently than once a month).
Aciclovir is effective only during the period of intake and has no residual beneficial effect. It does not eradicate the body viral pool. Following cessation of therapy, the time to onset of recurrences, their frequency, severity and duration remain generally unaffected. Some patients may experience increased severity of the first episode following cessation of therapy.
The risk of inducing viral resistance and of potential long-term adverse effects (see Section 5.3 Preclinical Safety Data) should be weighed carefully before initiating suppressive therapy.
Asymptomatic cases of genital herpes are known to shed the virus with a high frequency. However, at present only limited data are available on the extent and frequency of viral shedding in patients receiving suppressive therapy. Therefore, if therapy with aciclovir tablets is being used in the prenatal period (see Section 4.6 Fertility, Pregnancy and Lactation, Use in pregnancy), it should not be assumed that viral shedding has ceased. Pregnancy should be managed according to considerations normally applicable to patients with genital herpes.
In view of the complex and variable natural history of genital herpes, suppressive therapy should be interrupted periodically to ascertain whether the disease has undergone spontaneous change in frequency or severity (see Section 4.2 Dose and Method of Administration).
Intermittent treatment.
For certain patients, intermittent short-term treatment of recurrences is effective. Although the average patient would derive limited benefits from such treatment, a minority of patients who have experienced severe, prolonged recurrent episodes or recurrences complicated by eczema, burns or immunosuppression may experience more appreciable benefits. In those patients, intermittent treatment may be more appropriate than suppressive therapy when recurrences are infrequent.
Herpes zoster.
In controlled trials aciclovir tablets were shown to reduce acute pain and rash progression in adult patients of all ages with herpes zoster in whom the duration of rash was less than 72 hours. Aciclovir tablets appeared to be relatively less effective in younger adults, in whom herpes zoster is generally a milder disease.
In ophthalmic zoster, oral aciclovir has been shown to reduce the incidence of stromal keratitis and both the incidence and severity of anterior uveitis, but not other ocular complications or acute pain.
Note.
In immunocompetent patients with very severe herpes zoster, immunocompromised patients, or in patients with impaired absorption from the gut, consideration should be given to intravenous dosing.
Advanced symptomatic HIV disease.
Studies have shown that oral aciclovir reduced mortality in patients with advanced HIV disease (CD4+ counts < 150 x 106/L). In addition, oral aciclovir provided effective prophylaxis for herpes virus disease. No significant effect was seen on the prophylaxis of cytomegalovirus (CMV) disease or Epstein-Barr virus (EBV) disease.4.2 Dose and Method of Administration
Aciclovir-WGR tablets may be dispersed in a minimum of 50 mL of water, or swallowed whole with a glass of water.
Treatment of initial genital herpes.
One 200 mg tablet every four hours while awake, for a total of 5 tablets daily for ten days (total 50 tablets).
Chronic suppressive therapy for recurrent genital herpes.
One 200 mg tablet three times daily for up to six months. Many patients will, however, respond satisfactorily to one 200 mg tablet twice daily. Occasional breakthroughs have been reported in patients receiving 2, 3, 4 or 5 tablets daily. Suppressive therapy is not indicated for all patients with recurrent genital herpes (see Section 4.1 Therapeutic Indications). Therapy should be discontinued at the end of six months to ascertain whether any change has occurred in the natural course of the disease in the particular patient.
Intermittent therapy for recurrent genital herpes in certain patients.
(See Section 4.1 Therapeutic Indications). One 200 mg tablet every four hours while awake, for a total of 5 tablets daily for five days (total 25 tablets). Therapy should be initiated at the earliest sign or symptom (prodrome) of recurrence.
Treatment of herpes zoster in adults.
800 mg five times daily at approximately four hourly intervals, omitting the night-time dose. Therapy should commence as early as possible after the onset of rash but definitely within 72 hours of the appearance of the rash. Treatment should be continued for seven days. For herpes zoster ophthalmicus, the recommended duration of therapy is seven to ten days. Attention should be given to maintaining adequate hydration in elderly patients.
Management of patients with advanced symptomatic HIV disease.
800 mg four times daily at approximately six hourly intervals. The duration of treatment in the controlled trials was 12 months. Oral aciclovir was given in conjunction with oral zidovudine in most studies, at a range of doses. In a high percentage of the patients in the controlled trials, an initial zidovudine dose of 2 g daily followed after four weeks by 1 g daily was used. These doses are above the currently recommended dose of 600 mg daily. The safety and effectiveness of oral aciclovir taken in conjunction with other antiretroviral therapies could not be assessed.
Patients with acute or chronic renal impairment.
No data are currently available on the kinetics of oral aciclovir in patients with impaired renal function. However, based on studies with intravenous aciclovir infusion and theoretical considerations, the following dosage adjustments are recommended.
Genital herpes.
For patients with creatinine clearance < 10 mL/minute/1.73 m2, a 200 mg dose every twelve hours is recommended.
Herpes zoster and in the management of patients with advanced symptomatic HIV disease.
For patients with creatinine clearance in the range 10-25 mL/minute/1.73 m2, it is recommended to adjust the dosage to 800 mg three times daily (approximately every eight hours). For patients with creatinine clearance < 10 mL/minute/1.73 m2, 800 mg twice daily (approximately every twelve hours).
Dosage in the elderly.
The possibility of renal impairment in the elderly must be considered and the dosage should be adjusted accordingly (see above). Adequate hydration of elderly patients taking high oral doses of aciclovir should be maintained.4.3 Contraindications
Known hypersensitivity to aciclovir or valaciclovir.
4.4 Special Warnings and Precautions for Use
Use in renal impairment and in the elderly.
Aciclovir is eliminated by renal clearance, therefore the dose must be reduced in patients with renal impairment (see Section 4.2 Dose and Method of Administration). Elderly patients are likely to have reduced renal function and therefore the need for dose reduction must be considered in this group of patients. Both elderly patients and patients with renal impairment are at increased risk of developing neurological side effects and should be closely monitored for evidence of these effects. In the reported cases, these reactions were generally reversible on discontinuation of treatment (see Section 4.8 Adverse Effects (Undesirable Effects)). The dosage should be adjusted in patients with renal impairment (see Section 4.2 Dose and Method of Administration).
Hydration status.
Care should be taken to maintain adequate hydration in patients receiving high oral doses of aciclovir.
Other.
Resistant strains have been isolated in vitro and in animals following treatment with aciclovir. HSV strains resistant in vitro to aciclovir have also been isolated from immunocompromised as well as immunocompetent patients receiving aciclovir for herpes simplex infections. Therefore, the potential for the development of resistant HSV strains in patients treated with aciclovir should be borne in mind. The relationship between the level of in vitro sensitivity of herpes viruses to aciclovir and clinical response to therapy has not been adequately established.
As aciclovir has been associated with reversible encephalopathic changes, it should be used with caution in patients with underlying neurological abnormalities, significant hypoxia or serious renal, hepatic or electrolyte abnormalities. It should also be used with caution in patients who have manifested neurological reactions to cytotoxic drugs or are receiving interferon or intrathecal methotrexate concomitantly.
Animal studies indicate that at high doses aciclovir is cytotoxic.
Use in the elderly.
See Use in renal impairment and in the elderly.
Paediatric use.
Safety and effectiveness in children have not been established.
Effects on laboratory tests.
No data available.4.5 Interactions with Other Medicines and Other Forms of Interactions
Aciclovir is eliminated primarily unchanged in the urine via active renal tubular secretion. Any drugs administered concurrently that compete with this mechanism may increase aciclovir plasma concentrations. Probenecid and cimetidine increase the AUC of aciclovir by this mechanism, and reduce aciclovir renal clearance. However, no dosage adjustment is necessary because of the wide therapeutic index of aciclovir.
In patients receiving aciclovir, caution is required during concurrent administration with drugs which compete with aciclovir for elimination, because of the potential for increased plasma levels of one or both drugs or their metabolites. Increase in plasma AUCs of aciclovir and of the inactive metabolite of mycophenolate mofetil, an immunosuppressant agent used in transplants, have been shown when the drugs are coadministrated. However, no dosage adjustment is necessary because of the wide therapeutic index of aciclovir.
In patients over 60 years of age concurrent use of diuretics increases plasma levels of aciclovir very significantly. It is not known whether a similar effect occurs in young adults. In patients receiving zidovudine no significant overall increase in toxicity was associated with the addition of aciclovir. No data are available on interactions between aciclovir and other antiretroviral therapies.
4.6 Fertility, Pregnancy and Lactation
Effects on fertility.
There is no experience of the effect of aciclovir on human female fertility. In a study of 20 male patients with normal sperm count, oral aciclovir administered at doses of up to 1 g per day for up to six months has been shown to have no clinically significant effect on sperm count, motility or morphology.
(Category B3)
Animal studies show that aciclovir crosses the placenta readily. Aciclovir was not teratogenic in the mouse (450 mg/kg/day orally), rabbit (50 mg/kg/day subcutaneously and intravenously) or rat (50 mg/kg/day subcutaneously) when dosed throughout the period of major organogenesis. This exposure in the rat resulted in plasma levels 11-fold the mean steady state peak concentration in human doses of 800 mg every four hours. In additional studies in which rats were given three subcutaneous doses of aciclovir 100 mg/kg on gestation day 10, fetal abnormalities, e.g. head and tail anomalies, were reported (exposure was 63-fold human levels after 800 mg every four hours).
There have been no adequate and well controlled studies concerning the safety of aciclovir in pregnant women. It should not be used during pregnancy unless the benefits to the patient clearly outweigh the potential risks to the fetus. If suppressive therapy is used in the perinatal period, it should not be assumed that viral shedding has ceased, or that the risk to fetus/neonate has decreased. Pregnancy should be managed according to considerations normally applicable to patients with genital herpes.
Limited human data show that aciclovir does pass into breast milk. Aciclovir should only be administered to nursing mothers if the benefits to the mother outweigh the potential risks to the baby. Caution is therefore advised if acyclovir is to be administered to a nursing woman.4.7 Effects on Ability to Drive and Use Machines
The clinical status of the patient and the adverse event profile of aciclovir should be borne in mind when considering the patient's ability to drive or operate machinery. There have been no studies to investigate the effect of acyclovir on driving performance or the ability to operate machinery. Further, a detrimental effect on such activities cannot be predicted from the pharmacology of the active substance.
4.8 Adverse Effects (Undesirable Effects)
Aciclovir tablets appear to be generally very well tolerated. Adverse effects are usually mild. However, the following have been noted.
Short-term administration for treatment for genital herpes.
Nausea and/or vomiting and headache were the most frequent adverse effects. Less frequent (< 1%) reactions included diarrhoea, dizziness, anorexia, fatigue, oedema, skin rashes, leg pain, inguinal adenopathy, medication taste and sore throat. Occasional changes in liver enzymes and changes in haematological parameters were also noted.
Long-term suppressive therapy for genital herpes.
Nausea and/or vomiting, headache, diarrhoea, vertigo and arthralgia were the most frequent adverse effects. Less frequent adverse effects included skin rash, insomnia, fatigue, fever, palpitation, sore throat, superficial thrombophlebitis, muscle cramps, pars planitis, menstrual abnormalities, lymphadenopathy, irritability, accelerated hair loss, depression and occasional increases in liver enzymes.
Treatment of herpes zoster.
The most commonly reported adverse effect in clinical trials was gastrointestinal disturbance. Other reports included aching, chest pain, confusion, constipation, diarrhoea, giddiness, hallucinations, headache, insomnia, nausea, rash, shaking, taste disturbance, tremor, vertigo and malaise, vomiting and mental status alteration. Significantly, the overall incidence of side effects reported was the same in patients on placebo.
Patients with advanced symptomatic HIV disease.
In patients receiving antiretroviral therapy (mainly oral zidovudine), no significant overall increase in toxicity was associated with the addition of aciclovir. However, moderate increases in anaemia and neutropenia were seen in some studies in patients with advanced HIV disease.
The frequency categories associated with the adverse events below are estimates. For most events, suitable data for estimating incidence were not available. In addition, adverse events may vary in their incidence depending on the indication.
The following convention has been used for the classification of undesirable effects in terms of frequency: Very common ≥ 1/10, common ≥ 1/100 and < 1/10, uncommon ≥ 1/1000 and < 1/100, rare ≥ 1/10,000 and < 1/1000, very rare < 1/10,000.
Blood and lymphatic system disorders.
Very rare: anaemia, leukopenia, thrombocytopenia.
Immune system disorders.
Rare: anaphylaxis.
Psychiatric and nervous system disorders.
Common: headache, dizziness, confusion, hallucinations, somnolence, convulsions.
Very rare: agitation, tremor, ataxia, dysarthria, psychotic symptoms, encephalopathy, coma.
The above events are reversible and usually reported in patients with renal impairment in whom the dosage was in excess of that recommended, or with other predisposing factors.
Respiratory, thoracic and mediastinal disorders.
Rare: dyspnoea.
Gastrointestinal disorders.
Common: nausea, vomiting, diarrhoea, abdominal pains.
Hepato-biliary disorders.
Rare: reversible rises in bilirubin and liver related enzymes.
Very rare: hepatitis, jaundice.
Skin and subcutaneous tissue disorders.
Common: pruritus, rashes (including photosensitivity).
Uncommon: urticaria. Accelerated diffuse hair loss.
Accelerated diffuse hair loss has been associated with a wide variety of disease processes and medicines, the relationship of the event to aciclovir therapy is uncertain.
Rare: angioedema.
Renal and urinary disorders.
Rare: increases in blood urea and creatinine.
Very rare: acute renal failure, renal pain.
Renal pain may be associated with renal failure.
General disorders and administration site conditions.
Common: fatigue, fever.
Reporting suspected adverse effects.
Reporting suspected adverse reactions after registration of the medicinal product is important. It allows continued monitoring of the benefit-risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions at http://www.tga.gov.au/reporting-problems.4.9 Overdose
Symptoms and signs.
Aciclovir is only partly absorbed in the gastrointestinal tract. Patients have ingested overdoses of up to 20 g aciclovir on a single occasion, usually without toxic effects. Accidental, repeated overdoses of oral aciclovir over several days have been associated with gastrointestinal effects (such as nausea and vomiting) and neurological effects (headache and confusion).
Overdosage of intravenous aciclovir has resulted in elevations of serum creatinine, blood urea nitrogen and subsequent renal failure. Neurological effects including confusion, hallucinations, agitation, seizures and coma have been described in association with intravenous overdosage.
Management.
Patients should be observed closely for signs of toxicity. Adequate hydration is essential to reduce the possibility of crystal formation in urine. Haemodialysis significantly enhances the removal of aciclovir from the blood and may, therefore, be considered a management option in the event of symptomatic overdose.
For information on the management of overdose, contact the Poisons Information Centre on 13 11 26 (Australia).5 Pharmacological Properties
5.1 Pharmacodynamic Properties
Mechanism of action.
Microbiology.
Aciclovir is an antiviral agent which is active in vitro against HSV types I and II and varicella zoster virus (VZV), the latter being considerably less sensitive. The relationship between the level of in vitro sensitivity of herpes viruses to aciclovir and clinical response to therapy has not been adequately established. Development of resistance by HSV to aciclovir has been documented. Aciclovir needs to be phosphorylated to the active compound, aciclovir triphosphate, in order to become active against the virus. Such conversion is very limited in normal cells and, in addition, cellular DNA polymerase is not very sensitive to the active compound. However in infected cells, HSV or VZV coded thymidine kinase facilitates the conversion of aciclovir to aciclovir monophosphate, which is then converted to aciclovir triphosphate by cellular enzymes. Aciclovir triphosphate acts as an inhibitor of and substrate for the herpes specified DNA polymerase, preventing further viral DNA synthesis.
Clinical trials.
No data available.
5.2 Pharmacokinetic Properties
Aciclovir is only partially and variably absorbed from the gut. Estimated bioavailability following a dose of 200 mg is about 20% and decreases to about half of this with an 800 mg dose. Mean steady state peak and trough concentrations during dosage of 200 mg administered every four hours were 0.49 (range 0.47-0.54) microgram/mL and 0.31 (range 0.18-0.41) microgram/mL respectively, and after 800 mg six hourly were 1.43 (range 0.66-1.8) microgram/mL and 0.55 (range 0.14-1.10) microgram/mL respectively. Both peak and trough levels following repeated doses in adults over 60 years of age are considerably higher than in young adults, apparently because of the reduced renal function in the elderly.
Following oral administration of 200 mg aciclovir as Aciclovir-WGR 200 mg, the mean plasma half-life of aciclovir in volunteers with normal renal function was 3.4 hours. For volunteers dosed with 800 mg aciclovir as Aciclovir-WGR 800 mg, the mean plasma half-life was 7.2 hours.
Approximately 60% of the drug is excreted unchanged by the kidney by glomerular filtration and tubular excretion. When aciclovir is given after probenecid, the terminal half-life and the area under the plasma concentration-time curve are extended. 9-carboxymethoxymethyl guanine is the major metabolite of aciclovir and accounts for 10-15% of the dose excreted in the urine following intravenous administration.
In children aged 0 to 3 months the terminal plasma half-life is approximately 4 hours. However, experience is insufficient at present to recommend therapy for this age group.
Because aciclovir is excreted mainly by the kidneys, its total body clearance in the elderly (> 60 years of age) declines due to decreased renal function. The terminal half-life of aciclovir in the elderly is approximately 4.6 hours. It is important to maintain adequate hydration in elderly patients taking high oral doses.
In patients with chronic renal failure, the mean terminal half-life following intravenous administration was found to be 19.5 ± 5.9 (standard deviation) hours. The mean aciclovir half-life during haemodialysis was 5.7 hours. Plasma aciclovir levels dropped approximately 60% during dialysis.
Studies have shown no apparent changes in the pharmacokinetic properties of aciclovir or zidovudine when both are administered simultaneously to HIV infected patients.
Dosage adjustment for aciclovir tablets is recommended in renal impairment (see Section 4.2 Dose and Method of Administration).
Plasma protein binding is low (9-33%).
5.3 Preclinical Safety Data
Genotoxicity.
Aciclovir was clastogenic in Chinese hamster cells in vivo, at exposure levels also causing nephrotoxicity (500 and 1000 mg/kg parenteral dose). There was also an increase, though not statistically significant, in chromosomal damage at maximum tolerated doses (100 mg/kg) of aciclovir in rats. No activity was found in a dominant lethal study in mice or in four microbial assays. Positive results were obtained in two of seven genetic toxicity assays using mammalian cells in vitro (positive in human lymphocytes in vitro and one locus in mouse lymphoma cells, negative at two other loci in mouse lymphoma cells, and three loci in a Chinese hamster ovary cell line).
The results of these mutagenicity tests in vitro and in vivo suggest that aciclovir is unlikely to pose a genetic threat to humans at therapeutic dose levels.
Carcinogenicity.
Aciclovir was positive in one of two mouse cell transformation systems in vitro. Inoculation of the transformed cells into immunosuppressed mice resulted in tumours. These data are suggestive of an oncogenic potential. However, the validity of this type of study is unclear.
Lifetime oral dosing studies in mice and rats gave no evidence of tumorigenicity but in these species the absorption of oral aciclovir is poor and possibly self-limiting.6 Pharmaceutical Particulars
6.1 List of Excipients
Magnesium stearate, microcrystalline cellulose, sodium starch glycollate, pregelatinised maize starch, colloidal anhydrous silica.
6.2 Incompatibilities
Incompatibilities were either not assessed or not identified as part of the registration of this medicine.
6.3 Shelf Life
In Australia, information on the shelf life can be found on the public summary of the Australian Register of Therapeutic Goods (ARTG). The expiry date can be found on the packaging.
6.4 Special Precautions for Storage
Store below 25°C. Protect from light and moisture.
6.5 Nature and Contents of Container
200 mg tablets are available in blister packs of 50s and 90s.
800 mg tablets are available in blister packs of 35s.
6.6 Special Precautions for Disposal
In Australia, any unused medicine or waste material should be disposed of by taking to your local pharmacy.
6.7 Physicochemical Properties
Chemical structure.
 Chemical name: 2-amino-9-[(2-hydroxyethoxy) methyl]-1,9-dihydro- 6H-purin-6-one.
Chemical name: 2-amino-9-[(2-hydroxyethoxy) methyl]-1,9-dihydro- 6H-purin-6-one.
Synthetic acyclic purine nucleoside analogue. It is a white or almost white crystalline powder, slightly soluble in water, very slightly soluble in ethanol (96 per cent), practically insoluble in heptane. It dissolves in dilute solutions of mineral acids and alkali hydroxides.
CAS number.
59277-89-3.7 Medicine Schedule (Poisons Standard)
S4 - Prescription Only Medicine.
Summary Table of Changes


 Chemical name: 2-amino-9-[(2-hydroxyethoxy) methyl]-1,9-dihydro- 6H-purin-6-one.
Chemical name: 2-amino-9-[(2-hydroxyethoxy) methyl]-1,9-dihydro- 6H-purin-6-one.