What is in this leaflet
This leaflet answers some common questions about ADENURIC.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risk of you taking ADENURIC against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What ADENURIC is used for
ADENURIC is used in the treatment of long-standing high uric acid levels in the blood.
High levels of uric acid in the blood lead to development of crystals which deposit in the joints causing pain, swelling and tenderness. This is known as a condition called gout.
ADENURIC contains the active ingredient febuxostat.
It works by reducing the formation and accumulation of uric acid in the blood to reduce crystal formation. ADENURIC is used to prevent gout, but is not used to treat acute attacks of gout (also called flares).
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
This medicine is not addictive.
It is available only with a doctor's prescription.
There is not enough information to recommend the use of this medicine for children under the age of 18 years.
Before you use ADENURIC
When you must not take it
Do not take ADENURIC if you have an allergy to:
- any medicine containing febuxostat
- any of the ingredients listed at the end of this leaflet
Some symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin
Do not take this medicine if you are having an acute attack of gout and are not taking ADENURIC already. Your doctor will wait until the symptoms of the acute attack of gout have passed before starting you on ADENURIC.
If you have a gout flare while you are taking ADENURIC, do not stop taking this medicine.
Do not take this medicine if you are pregnant. It is not known whether it may affect your developing baby if you take it during pregnancy.
Do not breast-feed if you are taking this medicine. It is not known if the active ingredient in ADENURIC passes into breast milk and there is a possibility that your baby may be affected.
Do not give this medicine to a child under the age of 18 years. The safety and efficacy in children younger than 18 years old has not been established.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you have any allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you have or have had any of the following medical conditions:
- heart disease, heart failure, a heart attack or a stroke
- kidney problems
- allergic reaction to allopurinol (a medicine used to treat gout)
- liver problems
- high uric acid levels as a result of cancer or Lesch-Nyhan syndrome
- thyroid problems
- lactose intolerance
- organ transplant.
Tell your doctor if you are pregnant or plan to become pregnant or are breast-feeding. Your doctor can discuss with you the risks and benefits involved.
If you have not told your doctor about any of the above, tell them before you start taking ADENURIC. Your doctor will consider your cardiovascular risk factors before prescribing ADENURIC.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food store.
Some medicines and ADENURIC may interfere with each other. These include:
- mercaptopurine, a medicine used to treat cancer
- azathioprine, a medicine used to treat certain immune system problems
- theophylline, a medicine to treat asthma and other respiratory disorders
- tacrolimus, a medicine used to suppress the immune system after an organ transplant
- phenytoin, a medicine used to treat epilepsy.
These medicines may be affected by ADENURIC, or may affect how well it works. You may need different amounts of your medicine, or you may need to take different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
Ask your doctor or pharmacist if you are not sure if you are taking any of these medicines.
How to take ADENURIC
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
How much to take
The usual dose is 40 mg or 80 mg once daily. A starting dose of 40mg (half a tablet) is recommended and if your blood uric acid remains high after 2 to 4 weeks, the dose may be increased to 80 mg (1 whole tablet) once daily.
Your doctor will tell you how much to take. This depends on your condition and whether or not you are taking any other medicine.
How to take it
40 mg Dose:
You will need to break a tablet in half.
To break the tablet, hold the tablet between your thumbs and index fingers close to the score line.
Then, with the score line facing you, apply enough pressure to snap the tablet apart. Alternatively, use a tablet or pill cutter available from pharmacies.
Swallow one half of the tablet with a full glass of water. Keep the other half of the tablet for your next dose.
80 mg Dose:
Take one whole tablet with a full glass of water.
When to take it
Take your medicine at about the same time each day. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
It does not matter if you take this medicine before or after food.
How long to take it
Continue taking your medicine for as long as your doctor tells you.
This medicine helps to control your condition, but does not cure it. It is important to keep taking your medicine even if you feel well.
If you forget to take it
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take it as soon as you remember, and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for the dose that you missed. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre on 13 11 26 (Australia) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much ADENURIC. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
While you are using ADENURIC
Things you must do
Stop taking ADENURIC immediately and contact your doctor or go to Accident and Emergency at your nearest hospital if you develop any of the following allergy symptoms. Sometimes a serious but rare allergic reaction may follow:
- rash, including blisters and nodules
- shedding of the skin and inner surfaces of body cavities, e.g. mouth and genitals, painful ulcers in the mouth and/or genital areas, accompanied by fever, sore throat and fatigue
- enlarged lymph nodes
- generalised skin rashes
- itchiness
- swelling of limbs and face
- difficulties in breathing.
These may be the first signs of a serious allergic reaction to ADENURIC. You may need urgent medical attention.
Your doctor may decide to permanently stop treatment with ADENURIC.
Tell your doctor immediately if you have an acute gout attack and you are already taking ADENURIC. Your doctor may prescribe other medicines to treat the symptoms of flares.
Keep taking ADENURIC even if you have a flare. You may experience more flares and maybe worsening pain during the initial period of treatment. This is due to the way that ADENURIC and this class of medicines work. It is important that you continue to take ADENURIC and don’t stop during a gout flare. Over time, gout flares will occur less often and be less painful if you keep taking ADENURIC.
Tell your doctor if you develop any of the following symptoms while you are taking ADENURIC. They may be signs of liver problems:
- yellowing of skin and eyes (jaundice)
- dark urine
- fatigue
- loss in appetite
- pain in the right upper abdomen.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking ADENURIC.
Tell any other doctors, dentists, and pharmacists who are treating you that you are taking this medicine.
If you become pregnant while taking this medicine, tell your doctor immediately.
Keep all of your doctor's appointments so that your progress can be checked. Your doctor may do some blood tests from time to time to make sure the medicine is working.
Things you must not do
Do not take ADENURIC to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop taking your medicine or lower the dosage without checking with your doctor.
Things to be careful of
Be careful driving or operating machinery until you know how ADENURIC affects you. This medicine may cause drowsiness, dizziness, pins and needles, and blurred vision in some people.
If you have any of these symptoms, do not drive, operate machinery or do anything else that could be dangerous.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking ADENURIC.
This medicine helps most people with gout, but it may have unwanted side effects in a few people. All medicines can have side effects.
Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by the following list of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- headache
- nausea
- diarrhoea
- rash
- increase in gout symptoms
- localised swelling (oedema).
The above list includes the more common side effects of your medicine. They are usually mild.
If any of the following happen, tell your doctor immediately or go to Accident and Emergency at your nearest hospital:
- signs of heart problems such as chest pain, shortness of breath, trouble breathing, dizziness, fainting, feeling lightheaded, rapid or irregular heartbeat, numbness or weakness on one side of your body, slurring of speech, sudden blurry vision or sudden severe headache.
- sudden signs of an allergic reaction such as rash, itching or hives on the skin, swelling of the face, lips, tongue or other parts of the body, shortness of breath, wheezing or trouble breathing
- severe skin reaction, with painful red areas, large blisters and peeling of layers of skin
- pinkish, itchy swellings on the skin
- fever
- muscle pain, tenderness or weakness
The above list includes very serious side effects. You may need urgent medical attention or hospitalisation. These side effects are very rare.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Other side effects not listed above may also occur in some people. Some of these side effects can only be found when your doctor does tests from time to time to check your progress.
After using ADENURIC
Storage
Keep your tablets in the pack until it is time to take them. If you take the tablets out of the pack they may not keep well.
Keep your tablets in a cool, dry place away from light where the temperature stays below 30°C.
Do not store ADENURIC or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
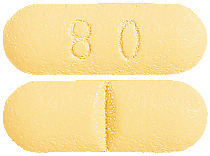
ADENURIC tablets are available in one strength of 80 mg.
The tablets are pale-yellow to yellow in colour and rectangular in shape. The tablets are scored on one side and marked "80" on the other side.
ADENURIC tablets are available in blister packs of 4, 8 or 28 tablets.
Not all pack sizes may be available.
Ingredients
ADENURIC contains febuxostat as the active ingredient.
Other ingredients include:
- lactose monohydrate
- microcrystalline cellulose
- magnesium stearate
- hyprolose
- croscarmellose sodium
- silicon dioxide.
Core tablets are coated with Opadry II Yellow 85F42129 containing:
- polyvinyl alcohol
- titanium dioxide
- macrogol 3350
- purified talc
- iron oxide yellow
ADENURIC does not contain sucrose, gluten, tartrazine or any other azo dyes.
Supplier
ADENURIC is supplied in Australia by:
A. Menarini Australia Pty Ltd
Level 8, 67 Albert Ave,
Chatswood, NSW, 2067
Medical Information: 1800 644 542
® = Registered Trademark
Australian Registration Number:
AUST R 205556
This leaflet was revised in December 2019
For the most up to date version of this leaflet, please go to www.menarini.com.au/cmi
[vD06-0]
Published by MIMS January 2020

 In a Phase IV post-authorisation CV safety study (FAST study) (see Section 5.1 Pharmacodynamic Properties, Clinical trials), the CV safety profile of febuxostat versus allopurinol in patients with chronic hyperuricaemia (in conditions where urate deposition had already occurred) and CV risk factors (i.e. patients 60 years or older and with at least one other CV risk factor) was investigated. In contrast to the CARES study where 100% of patients had a history of MACE, in the FAST study 66.6% of patients had no history of MACE.
In a Phase IV post-authorisation CV safety study (FAST study) (see Section 5.1 Pharmacodynamic Properties, Clinical trials), the CV safety profile of febuxostat versus allopurinol in patients with chronic hyperuricaemia (in conditions where urate deposition had already occurred) and CV risk factors (i.e. patients 60 years or older and with at least one other CV risk factor) was investigated. In contrast to the CARES study where 100% of patients had a history of MACE, in the FAST study 66.6% of patients had no history of MACE. The most common adverse reaction leading to discontinuation from therapy was liver function abnormalities in 1.8% of febuxostat 40 mg, 1.2% of febuxostat 80 mg and in 0.9% of allopurinol treated subjects.
The most common adverse reaction leading to discontinuation from therapy was liver function abnormalities in 1.8% of febuxostat 40 mg, 1.2% of febuxostat 80 mg and in 0.9% of allopurinol treated subjects.


 Table 7 summarises the efficacy endpoint results in all 3 studies.
Table 7 summarises the efficacy endpoint results in all 3 studies. The ability of Adenuric to lower serum uric acid levels was prompt and persistent. Reduction in serum uric acid level to < 357 micromole/L (6.0 mg/dL) was noted by the week 2 visit and was maintained throughout treatment.
The ability of Adenuric to lower serum uric acid levels was prompt and persistent. Reduction in serum uric acid level to < 357 micromole/L (6.0 mg/dL) was noted by the week 2 visit and was maintained throughout treatment.
 Overall, there were fewer deaths in the febuxostat group (62 of 3,063 CV deaths [0.61 per 100 patient years] and 108 of 3,063 all-cause deaths [1.06 per 100 patient years]), than in the allopurinol group (82 of 3,065 CV deaths [0.68 per 100 patient years] and 174 of 3,065 all-cause deaths [1.44 per 100 patient years]) (see Section 4.4 Special Warnings and Precautions for Use).
Overall, there were fewer deaths in the febuxostat group (62 of 3,063 CV deaths [0.61 per 100 patient years] and 108 of 3,063 all-cause deaths [1.06 per 100 patient years]), than in the allopurinol group (82 of 3,065 CV deaths [0.68 per 100 patient years] and 174 of 3,065 all-cause deaths [1.44 per 100 patient years]) (see Section 4.4 Special Warnings and Precautions for Use). Chemical name: 2-[3-cyano-4-(2-methylpropoxy)phenyl]-4-methylthiazole-5-carboxylic acid.
Chemical name: 2-[3-cyano-4-(2-methylpropoxy)phenyl]-4-methylthiazole-5-carboxylic acid.