What is in this leaflet
This leaflet answers to some common questions people ask about ADESAN HCT. It does not contain all the information that is known about ADESAN HCT.
It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor will have weighed the risks of you taking ADESAN HCT against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with this medicine. You may need to read it again.
What ADESAN HCT is used for
ADESAN HCT is used to treat high blood pressure.
Candesartan cilexetil is a type of medicine called an angiotensin II receptor antagonist (or blocker). It mainly works by causing relaxation of blood vessels.
Hydrochlorothiazide is a type of medicine called a diuretic. It works by reducing the amount of excess fluid in the body.
Using these two medicines together will lower your blood pressure more than using either one on its own.
Your doctor will have explained why you are being treated with ADESAN HCT and told you what dose to take.
Follow all directions given to you by your doctor carefully. They may differ from the information contained in this leaflet.
Your doctor may prescribe this medicine for another use. Ask your doctor if you want more information.
There is no evidence that ADESAN HCT is addictive.
Before taking ADESAN HCT
When you must not take it
Do not use ADESAN HCT if you have an allergy to:
- any medicine containing candesartan cilexetil, or hydrochlorothiazide
- any of the ingredients listed at the end of this leaflet
- any medicine containing an angiotensin II receptor antagonist (or blocker)
- any sulphur drugs (sulphonamides) such as some antibiotics or some medicines to treat diabetes
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin
Do not use ADESAN HCT if you have:
- severe kidney or liver disease and/or conditions associated with impaired bile flow (cholestasis)
- gout
Do not use ADESAN HCT if you are taking blood pressure medicine containing aliskiren, especially if you have diabetes mellitus or have kidney problems.
Do not use ADESAN HCT if you are pregnant or are planning to become pregnant. It may affect your baby if you take it during pregnancy.
Do not use ADESAN HCT if you are breastfeeding. Your baby can take in components of ADESAN HCT from breast milk if you are breastfeeding.
Do not give ADESAN HCT to children. There is no information about its use in children.
Do not use ADESAN HCT after the use by (expiry) date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to use it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you have any of the following medical conditions:
- kidney problems
- liver problems
- heart problems
- diabetes
- recent excessive vomiting or diarrhoea
- Systemic Lupus Erythematosus (SLE), a disease affecting the skin, joints and kidneys
- a salt restricted diet
- a condition called primary hyperaldosteronism
- a past operation known as sympathectomy
If you have not told your doctor about any of the above, tell him/her before you start taking ADESAN HCT.
Taking other medicines
Tell your doctor if you are taking any other medicines, including any that you get without a prescription from a pharmacy, supermarket or health food shop.
Some medicines and ADESAN HCT may interfere with each other. These include:
- Other blood pressure lowering medicines particularly diuretics (fluid tablets) and ACE-inhibitors, especially if you have diabetes-related kidney problems
- Medicines containing potassium, including salt substitutes
- Digoxin, a medicine used to treat heart failure
- Non-steroidal anti-inflammatory drugs (NSAIDs), medicines used to relieve pain, swelling and other symptoms of inflammation, including arthritis
- Colestipol and colestyramine, medicines used to treat high blood cholesterol levels
- Lithium, a medicine used to treat mood swings and some types of depression
- Alcohol
- Strong pain killers such as codeine, morphine, dextropropoxyphene
- Barbiturates, such as phenobarbital (phenobarbitone)
- Medicines like insulin that are used to treat diabetes
- Calcium supplements, or medicines containing calcium
- Vitamin D supplements
- Medicines to treat irregular heartbeats
- Corticosteroids such as prednisone, cortisone, dexamethasone
- Laxatives
- Medicines used to treat cancer such as cyclophosphamide monohydrate
- Methotrexate a medicine used to treat arthritis and some cancers
- Amantadine, a medicine used to treat Parkinson's disease
- Ciclosporin
These medicines may be affected by ADESAN HCT or may affect how it works. You may need different amounts of your medicines, or may need to take different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
How to take ADESAN HCT
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the label, ask your doctor or pharmacist for help.
How to take it
The usual dose is one tablet once daily, taken whole with a glass of water.
Take ADESAN HCT once a day, at about the same time each day. Keeping a regular time for taking ADESAN HCT will help to remind you to take it.
It does not matter whether you take ADESAN HCT with food or on an empty stomach.
How long to take it
ADESAN HCT helps control your condition, but does not cure it. Therefore you must take ADESAN HCT every day. Continue taking the tablets for as long as your doctor tells you to.
If you forget to take it
If you forget to take a dose, take it as soon as you remember, as long as it is at least 12 hours before your next dose is due. Then go back to taking it as you would normally.
If it is less than 12 hours to your next dose, skip the dose you missed and take your next dose when you are meant to.
Do not double the dose.
If you have trouble remembering when to take your medicine, ask your pharmacist for some hints.
If you take too much (Overdose)
Immediately telephone your doctor or the Australian Poisons Information Centre (telephone 13 11 26), or go to Accident and Emergency at your nearest hospital immediately if you think that you or anyone else may have taken too much ADESAN HCT even if there are no signs of discomfort or poisoning.
If you take too many ADESAN HCT tablets you may get a headache, feel sick (nausea), dizzy, thirsty and very tired.
While you are using ADESAN HCT
Things you must do
Take ADESAN HCT exactly as your doctor has told you to. Your blood pressure will not be well controlled if you do not.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking ADESAN HCT.
Tell your doctor if you have excessive vomiting or diarrhoea while taking ADESAN HCT. You may lose too much water and your blood pressure may become too low.
Tell all doctors, dentists and pharmacists who are treating you that you are taking ADESAN HCT.
Tell your doctor immediately if you become pregnant or plan to become pregnant while you are taking ADESAN HCT. You should not use ADESAN HCT if you are pregnant or thinking about becoming pregnant. Your doctor can discuss different treatment options with you.
If you plan to have surgery (even at the dentist) that needs a general anaesthetic, tell your doctor or dentist that you are taking ADESAN HCT.
Tell your doctor if you plan to have an examination such as an X-ray or a scan requiring an injection of iodinated contrast (dye) that you are taking ADESAN HCT.
If you have had skin cancer or if you develop a suspicious skin lesion during treatment with ADESAN PLUS, tell your doctor or pharmacist. Treatment with hydrochlorothiazide, particularly long-term use with high doses, may increase the risk of some types of skin and lip cancer (nonmelanoma skin cancer). Limit exposure to sunlight and protect your skin when exposed to sun while taking ADESAN PLUS.
Be sure to keep all of your doctor's appointments so that your progress can be checked. Your doctor will check your progress and may want to take some tests (e.g. blood tests, blood pressure) from time to time. These tests may help to prevent side effects.
Things you must not do
Do not use it to treat any other complaints unless your doctor says to.
Do not give this medicine to anyone else, even if they have symptoms that seem similar to yours.
Do not stop taking ADESAN HCT unless you have discussed it with your doctor.
Things to be careful of
Move slowly when getting out of bed or standing up if you feel faint, dizzy or light-headed.
Be careful driving or operating machinery until you know how ADESAN HCT affects you. You may feel dizzy when you start taking ADESAN HCT due to the drop in your blood pressure.
Drink plenty of water while you are using ADESAN HCT, especially if you sweat a lot.
Please talk to your doctor or pharmacist about these possibilities if you think they may bother you.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking ADESAN HCT.
ADESAN HCT helps most people with high blood pressure, but it may have unwanted side effects in a few people.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if you notice any of the following and they worry you:
- headache or dizziness
- flu-like symptoms or infections
- chest, throat or sinus infections
- feeling sick (nausea) or vomiting
- back pain
- urinary tract infection
- feeling tired
- stomach ache
- symptoms of sunburn which happens more quickly than normal.
These side effects are usually mild.
Tell your doctor as soon as possible if you notice any of the following:
- rapid heartbeats
- aching muscles, tenderness or weakness in the muscles
- suspicious skin lesions
The above list includes serious side effects that may require medical attention. Serious side effects are rare.
If any of the following happen, stop taking ADESAN HCT and tell your doctor immediately or go to Accident and Emergency at your nearest hospital.
- swelling of the face, lips, tongue or throat
- swelling of the hands, feet or ankles
- harsh sounds when breathing
- rash, itching or hives
- easy bruising or bleeding more easily than normal
- feel extremely tired
- yellowing of the skin and/or eyes
- signs of frequent infections such as fever, severe chills, sore throat or mouth ulcers
- worsening of the kidney function including passing little or no urine, drowsiness, nausea, vomiting, breathlessness, loss of appetite and weakness (especially in patients with existing kidney problems or heart failure)
- changes in your potassium, sodium and red or white blood cell levels may occur. Such changes are usually detected by a blood test
- symptoms that may indicate high potassium levels in the blood include nausea, diarrhoea, muscle weakness and changes in heart rhythm
These are very serious side effects. If you have them, you may have had a serious reaction to ADESAN HCT. You may need urgent medical attention or hospitalisation.
These side effects are very rare.
Tell your doctor if you notice anything else that is making you feel unwell.
Some people may get other side effects while taking ADESAN HCT.
After taking it
Storage
Keep your medicine in the blister pack until it is time to take them. If you take ADESAN HCT out of the blister pack it will not keep well.
Keep it in a cool, dry place and protect from light, where the temperature stays below 25C.
Do not store it or any other medicine in the bathroom or near a sink. Do not leave it on window sills or in the car on hot days. Heat and dampness can destroy some medicines.
Keep it where young children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
Ask your pharmacist what to do with any medicine you have left over if your doctor tells you to stop taking them, or you find that the expiry date has passed.
Product description
What ADESAN HCT looks like
ADESAN HCT 16/12.5 tablets are peach colour, mottled, round, biconvex tablet debossed with "M" on one side and "CH2" on the other.
ADESAN HCT 16/12.5 is available in blister packs of 7, 28 & 30's or bottle of 30 & 90's.
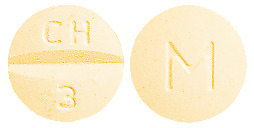
ADESAN HCT 32/12.5 tablets are yellow coloured, mottled, round, biconvex tablets debossed with "CH" above the break line and "3" below the break line on one side of the tablet and "M" on the other side.
ADESAN HCT 32/12.5 is available in blister packs of 7 & 30's or bottle of 7 & 30's.
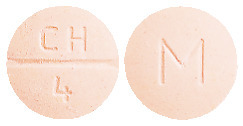
ADESAN HCT 32/25 tablets are peach coloured, mottled, round, biconvex tablets debossed with "CH" above the break line and "4" below the break line on one side of the tablet and "M" on the other side.
ADESAN HCT 32/25 is available in blister packs of 7 & 30's or bottle of 7 & 30's.
Ingredients
Each ADESAN HCT 16/12.5 tablet contains active ingredient candesartan cilexetil 16 mg and hydrochlorothiazide 12.5 mg.
Each ADESAN HCT 32/12.5 tablet contains active ingredient candesartan cilexetil 32 mg and hydrochlorothiazide 12.5 mg.
Each ADESAN HCT 32/25 tablet contains active ingredient candesartan cilexetil 32 mg and hydrochlorothiazide 25 mg.
The tablets also contain the following inactive ingredients:
- lactose monohydrate
- carmellose calcium
- glycerol monostearate
- hyprolose
- iron oxide yellow CI77492
- iron oxide red CI77491
- maize starch
- magnesium stearate
Supplier
ADESAN ® HCT is supplied by:
Alphapharm Pty Limited
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.mylan.com.au
Australian registration numbers:
ADESAN® HCT 16/12.5 mg tablets
- AUST R 163670 (Blisters)
- AUST R 163669 (Bottles)
ADESAN® HCT 32/12.5 mg tablets
- AUST R 206485 (Blisters)
- AUST R 206482 (Bottles)
ADESAN® HCT 32/25 mg tablets
- AUST R 206486 (Blisters)
- AUST R 206484 (Bottles)
This leaflet was prepared in July 2019.
ADESAN HCT_cmi\May19/00
Published by MIMS September 2019


 The following clinical adverse events occurred with a frequency of 0.5% to < 1% with no occurrence in the placebo group: A-V block, vomiting.
The following clinical adverse events occurred with a frequency of 0.5% to < 1% with no occurrence in the placebo group: A-V block, vomiting.
 Chemical name: (±)-1-(cyclohexyloxycarbonyl-oxy) ethyl 2-ethoxy-1-[[2'-(1H-tetrazol-5-yl) biphenyl-4-yl] methyl]-1H-benzimidazole-7- carboxylate.
Chemical name: (±)-1-(cyclohexyloxycarbonyl-oxy) ethyl 2-ethoxy-1-[[2'-(1H-tetrazol-5-yl) biphenyl-4-yl] methyl]-1H-benzimidazole-7- carboxylate. Chemical name: 6-chloro-3,4-dihydro- 2H-1,2,4-benzothiadiazine-7-sulphonamide 1, 1-dioxide.
Chemical name: 6-chloro-3,4-dihydro- 2H-1,2,4-benzothiadiazine-7-sulphonamide 1, 1-dioxide.