What is in this leaflet
This leaflet answers some common questions about Afinitor.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
The information in this leaflet was last updated on the date listed on the final page. More recent information on the medicine may be available.
You should ensure that you speak to your pharmacist or doctor to obtain the most up to date information on the medicine. You can also download the most up to date leaflet from www.novartis.com.au. Those updates may contain important information about the medicine and its use of which you should be aware.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking Afinitor against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What Afinitor is used for
Afinitor is used in the treatment of renal cell carcinoma, a type of kidney cancer; neuroendocrine tumours (NETs), a type of cancer located in the stomach and intestine, lung or pancreas; tuberous sclerosis complex (TSC) with angiomyolipoma of the kidney (a kidney tumour) not requiring immediate surgery; TSC with subependymal giant cell astrocytoma (sometimes called 'SEGA'), a specific type of brain tumour not requiring immediate surgery or certain types of seizures (epilepsy) associated with TSC in children and adults.
Afinitor is also used in the treatment of hormone receptor-positive HER2 negative advanced breast cancer in postmenopausal women, in conjunction with exemestane after failure of letrozole or anastrozole.
It is only used in patients whose tumour has tested negative to HER2.
Everolimus is the active substance in Afinitor.
Treatment of kidney cancer
Afinitor stops the cancer from making new cells and cuts off the blood supply. This slows the growth and spread of the cancer.
Treatment of NETs
Afinitor is used to control the growth of these tumours located in the stomach and intestine, lung or pancreas.
Treatment of SEGA
Afinitor reduces the size of brain tumours (SEGA) that are caused by a genetic disorder called tuberous sclerosis. This may stop the tumours from causing problems as they grow, such as hydrocephalus (excessive accumulation of fluid within the brain).
Treatment of TSC with angiomyolipoma of the kidney
Afinitor may reduce the size of angiomyolipoma of the kidney that is associated with a genetic disorder called TSC. This may lower the risk of the tumour(s) causing bleeding complications and may help to preserve kidney function.
Treatment of TSC with seizures
Afinitor may reduce the frequency of seizures in patients with a genetic disorder called TSC.
Treatment of hormone receptor-positive, HER2 negative advanced breast cancer
Growth of this type of breast cancer is stimulated by oestrogens which are female sex hormones. Oestrogen inhibitors such as letrozole, anastrozole and exemestane reduce the amount of oestrogen and can slow the growth of breast cancers. After failure of letrozole or anastrozole, exemestane is used in combination with Afinitor to prevent the breast cancer cells from becoming resistant to the exemestane.
The medicine is started by a specialist doctor experienced in tumour treatments.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
Afinitor is available only with a doctor's prescription and is not addictive.
Children and adolescents
AFINITOR is not to be used in children or adolescents for the treatment of hormone receptor-positive, HER2 negative advanced breast cancer, advanced neuroendocrine tumours or advanced kidney cancer.
AFINITOR is not to be used in children less than 1 years of age for treatment of SEGA associated with TSC.
Before you take Afinitor
When you must not take it
Do not take Afinitor if you have an allergy to:
- everolimus, the active ingredient in Afinitor
- a medicine called Rapamune which contains the active ingredient sirolimus
- any of the ingredients listed at the end of this leaflet
Some of the symptoms of an allergic reaction may include shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue or other parts of the body; rash, itching or hives on the skin.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you have or have had any of the following medical conditions:
- problems with your liver
- diabetes or high levels of blood sugar
Tell your doctor if you have any infections. It may be necessary to treat your infections before starting Afinitor.
Tell your doctor if you have previously had hepatitis B, because it may be reactivated during your treatment with Afinitor.
Tell your doctor if you have received or are about to receive radiation treatment.
Tell your doctor if you are pregnant or plan to become pregnant. Afinitor is not recommended for use during pregnancy. Your doctor can discuss with you the risks and benefits involved.
Tell your doctor if you are breast-feeding. Breastfeeding is not recommended while you are taking Afinitor and for two weeks after the last dose of Afinitor.. It is not known whether Afinitor passes into breast milk and could affect your baby.
Tell your doctor if you have lactose intolerance. Afinitor tablets or dispersible tablets contain lactose.
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes. Your doctor will want to know if you are prone to allergies.
Tell your doctor if you are about to have surgery or if you have had recent surgery or if you still have an unhealed wound following surgery. Afinitor may increase the risk of problems with wound healing.
If you have not told your doctor about any of the above, tell him/her before you start taking Afinitor.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and Afinitor may interfere with each other. These include:
- antibiotics such as rifampicin, rifabutin, clarithromycin and erythromycin
- antifungal medicines such as ketoconazole, voriconazole, fluconazole and itraconazole
- medicines for high blood pressure or heart problems such as diltiazem and verapamil
- medicines used to treat high blood pressure or other heart problems, known as ACE inhibitors
- drugs used to treat HIV/AIDS such as ritonavir, amprenavir, fosamprenavir, efavirenz and nevirapine
- epilepsy medicines such as carbamazepine, phenobarbitone and phenytoin
- St John's wort
- drugs used to stop the body from rejecting organ transplants such as ciclosporin
- drugs used to prevent vomiting such as aprepitant
- midazolam, a medicine used to treat acute seizures, or used as a sedative before or during surgery or a medical procedure
Such medicines may be affected by Afinitor or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
Tell your doctor if you are taking anti-seizure medications and your dose changes. A change in the dose of your medication (up or down) may require a change in Afinitor dose.
Tell your doctor if you need to receive a vaccination. Some vaccines may be less effective if given when taking Afinitor.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
How to take Afinitor
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the label ask your doctor or pharmacist for help.
How much to take
Treatment of kidney cancer, neuroendocrine tumours, hormone receptor positive, HER2 negative advanced breast cancer and TSC with angiomyolipoma of the kidney
Your doctor will tell you the dose that you should take. The usual dose is 10 mg taken once a day.
A higher or lower dose may be recommended in some situations (e.g. if you have liver problems).
Treatment of TSC with SEGA
Your doctor will tell you the dose that you need to take depending on your body size and other medicines you are taking.
Blood tests are necessary during treatment with Afinitor to measure the amount of Afinitor in your blood and find the appropriate daily dose for you.
Afinitor is not to be used in children and adolescents (below 18 years of age) who have liver problems.
Treatment of TSC with seizures
Your doctor will tell you the dose of Afinitor Dispersible Tablets you need to take depending on your body size, the health of your liver and other medicines you are taking. Blood tests are necessary during treatment with Afinitor to measure the amount of Afinitor in your blood and find the best daily dose for you. Follow carefully your doctor’s instructions about how much Afinitor to take.
Afinitor is not to be used in children below 2 years of age or in children and adolescents (below 18 years of age) who have liver problems.
How to take it
Take it consistently on an empty stomach or consistently after a light fat-free meal. Do not take it with a meal that is high in fat. Dietary fats can interfere with the absorption of the tablet or dispersible tablet and stop it working properly.
Do not take it with grapefruit juice. Grapefruit juice can interfere with the absorption of the tablet or dispersible tablet. Grapefruit, star fruit and Seville oranges should be avoided during treatment.
Afinitor Tablets
Swallow the tablet whole with a glass of water. Do not crush or chew it.
If you cannot swallow Afinitor tablets whole, you can stir them into a glass of water:
Put the required tablet(s) into a glass of water (containing approximately 30 mL)
Gently stir the contents until the tablet(s) break apart and drink immediately
Rinse the glass with the same amount of water (approximately 30 mL) and drink the contents to make sure that you get the full dose of Afinitor
Afinitor Dispersible Tablets
Do not chew, crush, or swallow the Afinitor Dispersible Tablets whole.
Take Afinitor Dispersible Tablets as a suspension only. You can prepare the suspension in an oral syringe or in a small drinking glass.
Oral syringe
- Remove the plunger from the oral syringe and put the prescribed number of dispersible tablets inside the 10mL syringe.
- Re-insert the plunger and push it inward to make contact with the dispersible tablets.
- Pull up about 5 mL of water into the syringe to cover the dispersible tablets. Then pull up about 4 mL of air.
- Place the filled syringe into a container (tip up) and allow the dispersible tablets 3 minutes to disintegrate.
- Gently turn the oral syringe upside down 5 times immediately prior to taking the medicine. Carefully remove the excess air and immediately dispense the full contents of the oral syringe directly into the mouth of the patient.
- After taking the medicine, pull up about 5mL of water and 4 mL of air into the same syringe and swirl the contents and then put the entire contents of the syringe into the mouth of the patient.
If you have any questions, contact your doctor or pharmacist.
Small drinking glass
- Add approximately 25 mL (2 tablespoons) of water into a small drinking glass and add the prescribed number of dispersible tablets.
- Allow the dispersible tablets to disintegrate for 3 minutes.
- Stir the contents of the glass gently with a spoon and immediately drink the full amount.
- Refill the glass with the same amount of water (about 25 mL), stir the contents with the same spoon to suspend remaining particles, and drink the entire content of the glass.
Instructions for use and handling of tablets and dispersible tablets
Caregivers are advised to avoid contact with suspensions of Afinitor tablets or dispersible tablets. Wash hands thoroughly before and after preparation of either suspension.
Take the tablet or dispersible tablet at the same time each day, preferably in the morning. Taking it at the same time each day will help you remember when to take it. It will also help to keep a steady amount of the medicine in your bloodstream.
How long to take it
Keep taking this medicine for as long as your doctor tells you.
If you forget to take it
If it is more than 6 hours after you normally take it, skip the tablet or dispersible tablet you missed and take your next tablet when you are meant to.
If it is less than 6 hours after you normally take it, you may still have the tablet or dispersible tablet. Then go back to taking your medicine as you would normally.
Do not take a double dose to make up for the dose that you missed. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much Afinitor. Do this even if there are no signs of discomfort or poisoning.
While you are taking Afinitor
Things you must do
Keep all of your doctor's appointments so that your progress can be checked. Your doctor will do tests from time to time to make sure the medicine is working and to prevent unwanted side effects.
You will have regular blood tests during treatment. These will monitor the amount of blood cells (white blood cells, red blood cells and platelets) in your body to see if Afinitor is having an unwanted effect on these cells. Blood tests will also be carried out to monitor your kidney function (levels of creatinine, blood urea nitrogen or urinary protein), liver function (level of transaminases) and your blood sugar levels, because these can also be affected by Afinitor.
Kidney failure has been observed in some patients receiving Afinitor. Your doctor will monitor your kidney function during your treatment with Afinitor.
Tell your doctor if you experience new or worsening cough, difficulty breathing, chest pain, shortness of breath or wheezing. Patients may develop lung or breathing problems, such as pneumonitis, pulmonary embolism or acute respiratory distress syndrome.
Your doctor may need to change the amount of Afinitor you have, or add another medicine to help with this side effect.
Tell your doctor straight away if you have a temperature or chills, or another sign of an infection. Afinitor can make you more likely to get an infection (such as infection of the lungs (pneumonia), infection of the urinary tract, fungal infection or viral infection such as infection of the liver (hepatitis B reactivation). You may need medical treatment. Some infections may be severe and may have fatal consequences in adults and children.
Tell your doctor if you experience pain or discomfort in the mouth or have open sores in the mouth. You might need treatment with a mouthwash, gel or other products. Some mouthwashes and gels can make ulcers worse, so do not try anything without checking with your doctor first.
Tell your doctor if you experience shortness of breath, nausea, diarrhoea, skin rashes or soreness in the mouth, gums or throat. These symptoms have been observed in patients receiving Afinitor along with radiation therapy or shortly after radiation therapy.
Make sure you use a contraceptive to prevent pregnancy during treatment with Afinitor. If you become pregnant while taking this medicine, tell your doctor immediately.
If you want to be vaccinated, tell your doctor you are taking Afinitor before you have the vaccination. Afinitor may affect your response to vaccination. Some vaccines may not be suitable for you.
Tell your doctor if you are about to have surgery or if you have had recent surgery or if you still have an unhealed wound following surgery. Afinitor can cause incisions to heal slowly or not heal well.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking Afinitor.
Tell any other doctors, dentists, and pharmacists who treat you that you are taking this medicine.
Things you must not do
Do not take Afinitor to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Things to be careful of
Be careful driving or operating machinery until you know how Afinitor affects you.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Afinitor.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- sore mouth, cold sores or mouth ulcers
- dry mouth
- dehydration
- fatigue or unusual weakness
- trouble sleeping (insomnia), aggression, irritability, feeling agitated, fits
- nausea, vomiting, diarrhoea, constipation, abdominal pain
- difficulty swallowing, loss of appetite, disturbance or loss of taste, weight loss
- heartburn
- headache, dizziness
- nose bleeds
- absence of periods (amenorrhea) or irregular periods in females
- swelling of hands, feet or limbs due to fluid retention
- dry or red skin
- rash and pain on the palms of your hands or soles of your feet
- infections including inflammation of the sinuses and nasal passages, middle or outer ear infection, gastric infection, sore throat and runny nose, skin infections, ringworm (a fungus infection of the skin), infections of the hair follicle, urinary tract infection, conjunctivitis, upper respiratory tract infection, pneumonia
- fever
- cough, stuffy or runny nose
- poor wound healing
- mouth ulcers
- nail disorders
- acne
- red, itching, oozing cysts which become scaly, crusted, or hardened
- small, solid pink or red bumps on the skin
- painful skin rash with small fluid-filled blisters in a limited area on one side of the body (left or right), often in a stripe (herpes zoster)
- headache, pressure in the eyes, nose or cheek area which is a sign of swelling of the sinuses and nasal area
- breathlessness
- bleeding e.g. in the gut wall
- cloudy urine
- trouble walking (gait disturbance).
If any of the following happen, tell your doctor immediately:
- symptoms of an allergic reaction such as shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue or other parts of the body; rash, itching or hives on the skin
- constant "flu-like" symptoms such as fever, chills, sore throat, aching joints and inflammation, swollen glands, cough, or any other signs of infection such as infection of a cut or scratch
- burning sensation on urination or increased urgency to urinate
- coughing, breathing problems, rapid breath or shortness of breath, which could be signs of a lung problem
- shortness of breath or rapid breathing which could be a sign of a lung problem
- fever, cough, difficulty breathing or wheezing
- shortness of breath, swelling of the feet or legs which could be a sign of heart failure
- sudden onset of shortness of breath, chest pain or coughing up blood which could be a sign of one or more blocked arteries in your lungs
- difficulty breathing, nausea, diarrhoea, soreness in the mouth, gums or throat, skin rashes or redness at site of radiation therapy
- symptoms of hepatitis B such as fever, skin rash, joint pain, inflammation, fatigue, loss of appetite, nausea, jaundice (yellowing of the skin), pain in the upper right abdomen, pale stool or dark urine
- high level of sugar in the blood (diabetes), worsening of diabetes
- swelling and/or pain in one of your legs, usually your calf, (redness or warm skin in the affected area)
- swelling, feeling of heaviness or tightness, pain, limited movement of body parts which could be a sign of fluid build-up and a problem with your lymphatic system.
The above list includes serious side effects. You may need urgent medical attention or hospitalisation.
Tell your doctor if you notice anything else that is making you feel unwell. Other side effects not listed here or not yet known may happen in some people. Some of these side effects can only be found by laboratory testing (for example, high levels of cholesterol, lipids, or sugar in the blood).
After taking Afinitor
Storage
- Keep the tablets or dispersible tablets in the original packet and foils until it is time to take them.
- Store the tablets or dispersible tablets in a cool, dark and dry place at room temperature.
- Do not store Afinitor or any other medicine in the bathroom or near a sink.
- Do not leave it in the car or on window sills.
Keep the medicine where young children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
Afinitor Tablets
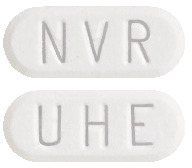
Afinitor tablets are white to yellowish and elongated with no score. Afinitor tablets are available in three different strengths, supplied in packs of 30, 50#, 60#, 100# and 120# tablets:
#not currently marketed
- 2.5mg: with "LCL" on one side and "NVR" on the other
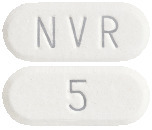
- 5 mg: with "5" on one side and "NVR" on the other
- 10 mg: with "UHE" on one side and "NVR" on the other
Afinitor Dispersible Tablets
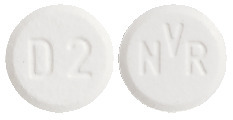
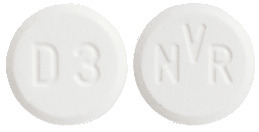
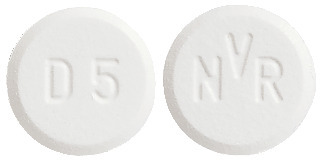
The dispersible tablets are white to slightly yellowish, round, flat tablets with a bevelled edge and no score.
- 2 mg dispersible tablet: with "D2" on one side and "NVR" on the other
- 3 mg dispersible tablet: with "D3" on one side and "NVR" on the other
- 5 mg dispersible tablet: with "D5" on one side and "NVR" on the other.
Ingredients
Afinitor Tablets
Afinitor tablets contain 2.5, 5 and 10 mg of everolimus as the active ingredient. They also contain:
- butylated hydroxytoluene
- magnesium stearate
- lactose monohydrate
- hypromellose
- crospovidone
- lactose.
Afinitor tablets contain sugars as lactose.
Afinitor Dispersible Tablets
Afinitor dispersible tablets contain 2, 3 and 5 mg of everolimus as the active ingredient. They also contain:
- butylated hydroxytoluene
- magnesium stearate
- lactose monohydrate
- hypromellose
- crospovidone
- mannitol
- microcrystalline cellulose
- colloidal anhydrous silica.
Afinitor tablets and dispersible tablets do not contain sucrose, gluten, tartrazine or any other azo dyes.
Sponsor
Afinitor is supplied in Australia by:
Novartis Pharmaceuticals Australia Pty Limited
ABN 18 004 244 160
54 Waterloo Road
Macquarie Park NSW 2113
Telephone 1 800 671 203
® = Registered Trademark
This leaflet was prepared in September 2021.
Afinitor Tablets
Australian Registration Number:
2.5 mg (AUST R 177648)
5 mg - (AUST R 154661)
10 mg - (AUST R 154663)
Afinitor Dispersible Tablets
2 mg - (AUST R 200203)
3 mg - (AUST R 200204)
5 mg - (AUST R 200205)
(afi230921c.doc) based on PI (afi230921i.doc)
Published by MIMS November 2021




 For example, a patient's current dose based on BSA is 4 mg with a steady state concentration of 4 nanogram/mL. In order to achieve a target concentration above the lower Cmin limit of 5 nanogram/mL, e.g. 8 nanogram/mL, the new everolimus dose would be 8 mg (an increase of 4 mg to the current daily dose). The trough concentration should then be assessed 1 to 2 weeks after this change in dose.
For example, a patient's current dose based on BSA is 4 mg with a steady state concentration of 4 nanogram/mL. In order to achieve a target concentration above the lower Cmin limit of 5 nanogram/mL, e.g. 8 nanogram/mL, the new everolimus dose would be 8 mg (an increase of 4 mg to the current daily dose). The trough concentration should then be assessed 1 to 2 weeks after this change in dose.

 Adverse drug reaction (ADR, assessed as related to treatment by the sponsor) information is based on pooled data from the 3 controlled studies mentioned above but including cumulative data (N = 608, including 408 patients < 18 years of age) from the double-blind plus open-label treatment phases and in addition from one non-randomised, open-label, single-arm phase II study (see Section 4.1 Therapeutic Indications; Table 5):
Adverse drug reaction (ADR, assessed as related to treatment by the sponsor) information is based on pooled data from the 3 controlled studies mentioned above but including cumulative data (N = 608, including 408 patients < 18 years of age) from the double-blind plus open-label treatment phases and in addition from one non-randomised, open-label, single-arm phase II study (see Section 4.1 Therapeutic Indications; Table 5): The most frequent ADRs (incidence ≥ 1/10, assessed as related to treatment by the sponsor) from the pooled safety database are (in decreasing order): stomatitis, pyrexia, nasopharyngitis, diarrhoea, upper respiratory tract infection, vomiting, cough, rash, headache, amenorrhea, acne, pneumonia, urinary tract infection, sinusitis, menstruation irregular, pharyngitis, decreased appetite, fatigue, hypercholesterolemia and hypertension.
The most frequent ADRs (incidence ≥ 1/10, assessed as related to treatment by the sponsor) from the pooled safety database are (in decreasing order): stomatitis, pyrexia, nasopharyngitis, diarrhoea, upper respiratory tract infection, vomiting, cough, rash, headache, amenorrhea, acne, pneumonia, urinary tract infection, sinusitis, menstruation irregular, pharyngitis, decreased appetite, fatigue, hypercholesterolemia and hypertension.
 Overall Survival (OS) data are not mature at the time of the interim analysis and no statistically significant treatment-related difference in OS was noted [HR = 0.77 (95% CI: 0.57, 1.04)].
Overall Survival (OS) data are not mature at the time of the interim analysis and no statistically significant treatment-related difference in OS was noted [HR = 0.77 (95% CI: 0.57, 1.04)]. Nine-month PFS rates were 44% of patients receiving Afinitor + exemestane compared with 16% in the placebo + exemestane arm at a median follow-up of 17.7 months.
Nine-month PFS rates were 44% of patients receiving Afinitor + exemestane compared with 16% in the placebo + exemestane arm at a median follow-up of 17.7 months.
 Clinically or statistically significant differences were not observed between the two treatment arms in terms of time to deterioration of ECOG PS (≥ 1 point) and median times to deterioration (≥ 5%) of QLQ-C30 domain scores.
Clinically or statistically significant differences were not observed between the two treatment arms in terms of time to deterioration of ECOG PS (≥ 1 point) and median times to deterioration (≥ 5%) of QLQ-C30 domain scores.
 Eighteen-months PFS rates were 34.2% for Afinitor therapy compared to 8.9% for placebo.
Eighteen-months PFS rates were 34.2% for Afinitor therapy compared to 8.9% for placebo.
 In supportive analyses, positive treatment effect has been observed in all subgroups with the exception of the subgroup of patients with ileum as primary site of tumour origin (Ileum: HR = 1.22 [95% CI: 0.56 to 2.65]; Non-ileum: HR = 0.34 [95% CI: 0.22 to 0.54]; Lung: HR = 0.43 [95% CI: 0.24 to 0.79]) (see Figure 6).
In supportive analyses, positive treatment effect has been observed in all subgroups with the exception of the subgroup of patients with ileum as primary site of tumour origin (Ileum: HR = 1.22 [95% CI: 0.56 to 2.65]; Non-ileum: HR = 0.34 [95% CI: 0.22 to 0.54]; Lung: HR = 0.43 [95% CI: 0.24 to 0.79]) (see Figure 6). The overall response rate as per independent assessment was 2% in the everolimus arm vs. 1% in the placebo arm. Disease control rate (CR or PR or SD) for everolimus was 82.4% vs. 64.9% in the placebo arm. Tumour reduction was also evident from the corresponding waterfall plot. Results indicate that 63.6% of patients in the everolimus arm experienced tumour shrinkage versus 25.9% for placebo (Figure 7).
The overall response rate as per independent assessment was 2% in the everolimus arm vs. 1% in the placebo arm. Disease control rate (CR or PR or SD) for everolimus was 82.4% vs. 64.9% in the placebo arm. Tumour reduction was also evident from the corresponding waterfall plot. Results indicate that 63.6% of patients in the everolimus arm experienced tumour shrinkage versus 25.9% for placebo (Figure 7). The final overall survival (OS) analysis did not show statistically significant difference between those patients who received Afinitor or placebo during the blinded treatment period of the study [HR = 0.90 (95% CI: 0.66 to 1.24)] (Figure 8).
The final overall survival (OS) analysis did not show statistically significant difference between those patients who received Afinitor or placebo during the blinded treatment period of the study [HR = 0.90 (95% CI: 0.66 to 1.24)] (Figure 8). Clinically or statistically significant differences were not observed between the two treatment arms in terms of time to deterioration of WHO PS (HR: 1.02; 95% CI: 0.65, 1.61) and time to deterioration of FACT-G total score (HR: 0.74; 95% CI: 0.50, 1.10).
Clinically or statistically significant differences were not observed between the two treatment arms in terms of time to deterioration of WHO PS (HR: 1.02; 95% CI: 0.65, 1.61) and time to deterioration of FACT-G total score (HR: 0.74; 95% CI: 0.50, 1.10).
 Six-month PFS rates were 36% for Afinitor therapy compared with 9% for placebo.
Six-month PFS rates were 36% for Afinitor therapy compared with 9% for placebo. See Table 11.
See Table 11. Percentage reduction from baseline in seizure frequency was a supporting analysis.
Percentage reduction from baseline in seizure frequency was a supporting analysis.
 Consistent treatment effects were observed across all subgroups evaluated (i.e. EIAED use vs. EIAED non-use, sex, age, and race) at the primary efficacy analysis (Table 14).
Consistent treatment effects were observed across all subgroups evaluated (i.e. EIAED use vs. EIAED non-use, sex, age, and race) at the primary efficacy analysis (Table 14). The waterfall plots provide a graphical representation of the reduction in angiomyolipoma volume (Figure 11) at primary analysis; 95.5% of patients in the Afinitor arm experienced angiomyolipoma shrinkage versus 59.4% in the placebo arm.
The waterfall plots provide a graphical representation of the reduction in angiomyolipoma volume (Figure 11) at primary analysis; 95.5% of patients in the Afinitor arm experienced angiomyolipoma shrinkage versus 59.4% in the placebo arm. In the final analysis, reduction in angiomyolipoma volume improved with longer term treatment with Afinitor. At weeks 12, 96 and 192, ≥ 30% reductions in volume were observed in 75.0% (78/104), 80.6% (79/98) and 85.2% (52/61) of the treated patients, respectively. Similarly, at the same timepoints, ≥ 50% reductions in volume were observed in 44.2% (46/104), 63.3% (62/98) and 68.9% (42/61) of the treated patients, respectively.
In the final analysis, reduction in angiomyolipoma volume improved with longer term treatment with Afinitor. At weeks 12, 96 and 192, ≥ 30% reductions in volume were observed in 75.0% (78/104), 80.6% (79/98) and 85.2% (52/61) of the treated patients, respectively. Similarly, at the same timepoints, ≥ 50% reductions in volume were observed in 44.2% (46/104), 63.3% (62/98) and 68.9% (42/61) of the treated patients, respectively.
 At the primary analysis, Afinitor demonstrated clinically meaningful and statistically significant improvements in skin lesion response (p = 0.0002), with response rates of 26.0% (20/77) (95% CI: 16.6, 37.2) for the Afinitor arm and 0% (0/37) (95% CI: 0.0, 9.5) for the placebo arm (Table 15). At the final analysis, the skin lesion response rate had increased to 68.2% (73/107) (95% CI: 58.5%, 76.9%) (Table 15), with one patient reporting a confirmed complete clinical skin lesion response and no patients experiencing progressive disease as their best response.
At the primary analysis, Afinitor demonstrated clinically meaningful and statistically significant improvements in skin lesion response (p = 0.0002), with response rates of 26.0% (20/77) (95% CI: 16.6, 37.2) for the Afinitor arm and 0% (0/37) (95% CI: 0.0, 9.5) for the placebo arm (Table 15). At the final analysis, the skin lesion response rate had increased to 68.2% (73/107) (95% CI: 58.5%, 76.9%) (Table 15), with one patient reporting a confirmed complete clinical skin lesion response and no patients experiencing progressive disease as their best response. In an exploratory analysis of patients with TSC with angiomyolipoma who also had SEGA, the SEGA response rate (proportion of patients with ≥ 50% reduction from baseline in target lesion volumes in the absence of progression) was 10.3% (4/39) in the everolimus arm at the primary analysis (versus no responses reported in the 13 patients randomised to placebo with a SEGA lesion at baseline) and increased to 48.0% (24/50) at the final analysis.
In an exploratory analysis of patients with TSC with angiomyolipoma who also had SEGA, the SEGA response rate (proportion of patients with ≥ 50% reduction from baseline in target lesion volumes in the absence of progression) was 10.3% (4/39) in the everolimus arm at the primary analysis (versus no responses reported in the 13 patients randomised to placebo with a SEGA lesion at baseline) and increased to 48.0% (24/50) at the final analysis. Consistent treatment effects were observed across all subgroups evaluated (i.e. EIAED use vs. EIAED non-use, sex, and age) at the primary analysis (Table 17).
Consistent treatment effects were observed across all subgroups evaluated (i.e. EIAED use vs. EIAED non-use, sex, and age) at the primary analysis (Table 17). During the double-blind period, reduction of SEGA volume was evident within the initial 12 weeks of treatment with Afinitor: 29.7% (22/74) of patients had ≥ 50% reductions in volume and 73.0% (54/74) of patients had ≥ 30% reductions in volume. Sustained reductions were evident at Week 24, 41.9% (31/74) of patients had ≥ 50% reductions and 78.4% (58/74) of patients had ≥ 30% reductions in SEGA volume.
During the double-blind period, reduction of SEGA volume was evident within the initial 12 weeks of treatment with Afinitor: 29.7% (22/74) of patients had ≥ 50% reductions in volume and 73.0% (54/74) of patients had ≥ 30% reductions in volume. Sustained reductions were evident at Week 24, 41.9% (31/74) of patients had ≥ 50% reductions and 78.4% (58/74) of patients had ≥ 30% reductions in SEGA volume.
 Additional clinical benefits of Afinitor were observed such as reductions in severity of skin lesions and size of renal angiomyolipoma.
Additional clinical benefits of Afinitor were observed such as reductions in severity of skin lesions and size of renal angiomyolipoma. At the time of the primary analysis, angiomyolipoma responses were only observed in the everolimus arm (n/N:16/30; 53.3%; 95% CI: 34.3, 71.7). At the time of final analysis, among the 41 TSC-SEGA patients with an angiomyolipoma lesion(s) present at start of treatment with everolimus, 30 patients (73.2%; 95% CI: 57.1, 85.8) achieved, as their best overall response, at least a 50% reduction in sum of angiomyolipoma volumes. Among the 37 patients with evaluable angiomyolipoma tumour assessments, 35 patients (94.6%) experienced a reduction in the sum of target angiomyolipoma volumes relative to baseline as their best percentage change. Over the entire duration of the study, no new angiomyolipoma lesions were observed, nor were instances of grade 2 or worse bleeding episodes reported.
At the time of the primary analysis, angiomyolipoma responses were only observed in the everolimus arm (n/N:16/30; 53.3%; 95% CI: 34.3, 71.7). At the time of final analysis, among the 41 TSC-SEGA patients with an angiomyolipoma lesion(s) present at start of treatment with everolimus, 30 patients (73.2%; 95% CI: 57.1, 85.8) achieved, as their best overall response, at least a 50% reduction in sum of angiomyolipoma volumes. Among the 37 patients with evaluable angiomyolipoma tumour assessments, 35 patients (94.6%) experienced a reduction in the sum of target angiomyolipoma volumes relative to baseline as their best percentage change. Over the entire duration of the study, no new angiomyolipoma lesions were observed, nor were instances of grade 2 or worse bleeding episodes reported. Long-term follow-up to a median duration of 67.8 months (range: 4.7 to 83.2 months) demonstrated sustained efficacy with a median reduction in primary SEGA volume per independent central review of 0.50 cm3 at month 60 (range: -0.74 to 9.84 cm3; n = 23).
Long-term follow-up to a median duration of 67.8 months (range: 4.7 to 83.2 months) demonstrated sustained efficacy with a median reduction in primary SEGA volume per independent central review of 0.50 cm3 at month 60 (range: -0.74 to 9.84 cm3; n = 23).
