What is in this leaflet
This leaflet answers some common questions about Airomir Autohaler. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you using Airomir Autohaler against the benefits they expect it will have for you.
If you have any concerns about using this medicine, ask your doctor or pharmacist.
Keep this leaflet with your medicine. You may need to read it again.
What's in Airomir Autohaler
The name of your medication is Airomir Autohaler. It contains a medicine called salbutamol sulfate. Salbutamol sulfate belongs to the beta-agonist family of medicines. The amount of medicine in each puff of Airomir Autohaler is the same as 100 micrograms of salbutamol. Airomir Autohaler contains at least 200 puffs.
What Airomir Autohaler is used for
Airomir Autohaler is designed so the medicine can be inhaled (breathed in) into the lungs to treat asthma and other conditions where breathing is difficult. Salbutamol sulfate opens up the airways to your lungs, thereby relieving wheezing and the feeling of tightness in your chest, and helps you breathe more easily. Airomir Autohaler is known as a RELIEVER medicine.
Your medicine may also help prevent wheezing and chest tightness in some situations that cause narrowing of the airways to your lungs, like exercise. That's why your doctor or pharmacist may advise you to take one or two puffs of Airomir Autohaler before exercise.
Your Airomir Autohaler is designed to be just one part of a general plan to help you manage your asthma. Every asthma patient is different. Ask your doctor or pharmacist for an ASTHMA MANAGEMENT PLAN that suits you. Make sure you visit your doctor or pharmacist regularly to check whether your plan needs to be changed.
Perhaps you have been prescribed Airomir Autohaler for something other than asthma.
Ask your doctor if you have any questions about why this medicine has been prescribed for you.
What's different about Airomir Autohaler?
If you have been using an older type of inhaler you will find that Airomir Autohaler has a different taste and a warm, soft spray. But it is still just as effective.
Each spray from Airomir Autohaler contains exactly the same dose of salbutamol sulfate active ingredient as a spray from the older type of inhaler.
Before using Airomir Autohaler
When you must not use it
Do not use Airomir Autohaler if you are allergic to:
- salbutamol and similar drugs
- any of the ingredients listed under "Product description" later in this leaflet
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin
Do not take this medicine after the expiry date printed on the canister or if the packaging is torn or shows signs of tampering.
If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start using this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor or pharmacist if you have or have had any of the following medical conditions:
- heart problems
- liver or kidney problems
- diabetes (high blood sugar)
- high blood pressure
- problems with your thyroid gland
- hypoxia (less oxygen in the tissues)
Tell your doctor or pharmacist if you are pregnant or plan to become pregnant or are breast-feeding. Your doctor or pharmacist can discuss with you the risks and benefits involved.
If you have not told your doctor or pharmacist about any of the above, tell them before you start using Airomir Autohaler.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and Airomir Autohaler may interfere with each other.
These include:
- any medications for high blood pressure or angina (for example atenolol, metoprolol, propranolol or pindolol)
- theophylline or any steroids
- fluid tablets (diuretics), (for example frusemide or chlorothiazide)
These medicines may be affected by Airomir Autohaler or may affect how well they work. You may need different amounts of your medicines, or you may need to take different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
How to use Airomir Autohaler
Carefully follow all directions given to you by your doctor or pharmacist. They may differ from the information contained in this leaflet.
Even if you have been using another type of inhaler or autohaler, please read the instructions for use in this leaflet before you start.
Your Airomir Autohaler may not help you as much as it should if not used correctly.
If you do not understand the instructions, ask your doctor or pharmacist for help.
Airomir Autohaler Instructions for Use
If your Airomir Autohaler is new or has not been used for two weeks or more, you must test fire it by releasing four puffs into the air away from your face.
- REMOVE COVER AND SHAKE
Remove the mouthpiece cover by unclipping it from the back.
Check that the mouthpiece is clean before use.
Hold the autohaler unit upright between your thumb and index finger and shake well. - HOLD UPRIGHT AND PUSH LEVER UP
Hold the autohaler unit upright.
Push the lever up so that it stays up. Keep holding the autohaler unit upright, making sure that your hand is not blocking the air vent at the bottom. - BREATHE OUT AND POSITION MOUTHPIECE.
Breathe out as far as you comfortably can and immediately close your lips around the mouthpiece. - BREATHE IN
slowly and deeply through the mouthpiece.
Do not stop breathing in when you hear the click and whoosh and feel the puff in your mouth. It is important that you keep breathing in after the puff is released - HOLD YOUR BREATH
for 10 seconds, then breathe out slowly. - PUSH LEVER DOWN
After each puff return the lever to the down position whilst holding the autohaler unit upright.
If your doctor has prescribed more than one puff, repeat steps 2 to 6. Replace the mouthpiece cover after use.
Remember to push the lever up before each puff, and gently back down afterwards, always holding the autohaler upright. This prepares the autohaler for your next dose. The lever should be left down between treatments and the mouthpiece cover replaced to keep your Airomir Autohaler clean.
How to test fire or tell if your Airomir Autohaler is empty
- REMOVE THE MOUTHPIECE COVER
by unclipping it from the back.
Shake the Autohaler. - Hold your Airomir Autohaler upright and point the mouth-piece away from you so that the puffs of medicine will go into the air.
PUSH THE LEVER UP
so that it stays up. - RELEASE A PUFF
by pushing the dose release slide on the bottom of the Autohaler unit in the direction of the arrow. - TO RELEASE THE SECOND PUFF
first return the lever to its down position, then repeat steps 2 and 3 above. Repeat until you have released four puffs altogether. - ALWAYS PUSH THE LEVER BACK DOWN
after test firing. This prepares the autohaler for your next dose. - If your Airomir Autohaler is empty you will not feel or hear a puff being discharged when you test fire.
Do not use the dose release slide to take your medicine. Your Airomir Autohaler will automatically release a dose when you begin to breathe in from the mouthpiece.
Airomir Autohaler is designed for ease of use. To make it work you just breathe in through the mouthpiece.
There is no need to press and breathe in at the same time. A click and whoosh tells you that Airomir Autohaler has automatically released the correct dose of medicine.
If your Airomir Autohaler does not work properly
When your autohaler is blocked little or no medicine comes out when you press down on the metal canister.
This may be for one of the following reasons:
- A dirty or clogged mouthpiece: Wash the mouthpiece as described in Step 1 and air dry thoroughly as described in Step 2.
- It may be empty. Check by shaking.
- It may be put together wrongly.
It is important that the canister in the correct position in your autohaler. The narrow stem of the metal canister should be fitted into the small socket.
If the stem of the metal canister is not in the small socket, your autohaler will not work.
Do not take your Airomir Autohaler apart.
Do not drop or hit your Airomir Autohaler because it could be damaged.
How much to use
Use Airomir Autohaler only as directed by your doctor or pharmacist.
Adults
The usual dose is one or two puffs to relieve wheezing, or before exercise.
You should not use more than 16 puffs per day without consulting your doctor.
Do not use Airomir Autohaler more often than your doctor or pharmacist tells you, or as stated in this leaflet.
Children
As for adults, or as directed by your doctor.
Children using Airomir Autohaler should be supervised by a responsible adult.
Elderly
Older people should use Airomir Autohaler as directed by their doctor.
How to take it
Carefully follow the instructions above under "How to use Airomir Autohaler".
If you use too much (overdose)
Do not use more than the recommended dose unless your doctor tells you to.
Too much use of any asthma medication may be dangerous. If you use more puffs than those recommended you may suffer unwanted effects. You may feel tense and shaky and your heart may beat faster than usual.
Immediately telephone your doctor or the Poisons Information Centre (in Australia call 131126; in New Zealand call 0800 764 766) for advice, or go to Accident and Emergency at the nearest hospital, if you think you or anyone else may have used too much Airomir Autohaler Do this even if there are no signs of discomfort or poisoning.
You may need medical attention.
While you are using Airomir Autohaler
Things you must do
If you are about to be started on any new medicine remind your doctor and pharmacist that you are taking Airomir Autohaler.
Tell any other doctors, dentists, and pharmacists who treat you that you are using this medicine.
If your usual dose of Airomir Autohaler does not seem to be working or it is not lasting as long as before, contact your doctor urgently.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking this medicine. It may affect other medicines used during surgery.
If you become pregnant while taking this medicine, tell your doctor immediately.
Things you must not do
Do not use Airomir Autohaler to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else even if they have the same condition as you.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are using Airomir Autohaler.
This medicine helps most people with asthma, but it may have unwanted side effects in a few people and may occur with the normal use of your Airomir Autohaler.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- fine shaking in the hands
- headache
- feeling tense
- nausea
- light headedness
- leg cramps
- dizziness
- chest pain
- palpitations (when your heart beats faster and harder than normal)
- lowering of the potassium level in the blood
- minor irritation to your mouth and throat
The above list includes the more common side effects of your medicine. They are usually mild and short-lived.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Other side effects not listed above may also occur in some people.
After using Airomir Autohaler
Care and Cleaning
Keeping the plastic mouthpiece clean is very important to prevent the autohaler from becoming dirty and clogged.
Your autohaler should be cleaned at least once a week as follows:
STEP 1.
WASH MOUTHPIECE UNDER WARM RUNNING WATER.
Remove the canister and put aside in a safe place. Never immerse the canister in water.
Wash the mouthpiece through the top and bottom with warm running water for 30 seconds.
Wash the mouthpiece cover as well.
STEP 2.
SHAKE OFF EXCESS WATER AND AIR DRY.
To dry, shake off excess water and leave the mouthpiece and cover in a safe place to air dry thoroughly, such as overnight.
When the mouthpiece is dry, replace the canister and the mouthpiece cover.
If you need to use your autohaler before it is completely dry, shake off excess water, replace the canister and remove most of the water remaining in the mouthpiece by test spraying twice into the air away from your face. Then take your dose as prescribed. After such use, wash the autohaler again and air dry thoroughly as recommended above.
NOTE:
Blockage from medication build-up is more likely to occur if the mouthpiece is not allowed to air-dry thoroughly.
Storage
Keep your Airomir Autohaler in a cool dry place where the temperature stays below 30°C.
Do not store Airomir Autohaler or any other medicine in the bathroom or near a sink. Do not leave it on a windowsill or in the car. Heat and dampness can destroy some medicines.
Keep it (together with the mouthpiece cover) in a place where children cannot reach. A locked cupboard at least one-and-one-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Dispose of your Airomir Autohaler thoughtfully, remembering that the metal canister is pressurised. Do not burn or puncture the metal canister even when empty, as it may explode.
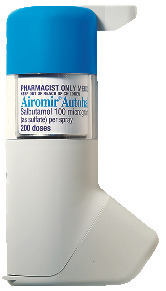
Product description
What it looks like
Airomir Autohaler (AUST R 67257) consists of a metal canister sealed inside a plastic autohaler unit. At the bottom of the autohaler unit there is a mouthpiece with a plastic cover. At the top of the autohaler unit is a lever. Available in pack sizes of 100* & 200 doses.
* Not currently marketed
Ingredients
Airomir Autohaler releases 100 micrograms of salbutamol in each puff.
Airomir Autohaler also contains:
- ethanol (0.0036 mL per puff)
- oleic acid
- norflurane - a non-CFC propellant that does not deplete ozone from the atmosphere.
Sponsor/Supplier
Airomir Autohaler is supplied by:
iNova Pharmaceuticals (Australia) Pty Limited
(ABN 13 617 871 539)
Level 10, 12 Help Street
Chatswood NSW 2067
Australian Toll Free: 1800 630 056
New Zealand Toll Free: 0508 375 394
Developed and manufactured by:
3M Health Care Limited
Loughborough UK
™ = Trademark
3M and Airomir are trademarks.
AeroChamberPlus is a registered trademark of Trudell Medical.
This leaflet was prepared in December 2017.
Published by MIMS February 2018


 (RS)-2-tert-butylamino- 1-(4-hydroxy- 3-hydroxymethylphenyl) ethanol sulfate.
(RS)-2-tert-butylamino- 1-(4-hydroxy- 3-hydroxymethylphenyl) ethanol sulfate.