What is in this leaflet
This leaflet answers some common questions about Aldactone.
It does not contain all of the available information.
It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking Aldactone against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What Aldactone is used for
What Aldactone does
Aldactone is used:
- To treat essential hypertension (high blood pressure with an unknown cause)
- To treat oedematous disorders (swelling with fluid), including congestive cardiac failure
- For the diagnosis and treatment of primary aldosteronism (a hormone disorder causing fluid retention)
- As add-on therapy in malignant hypertension (a very serious form of high blood pressure)
- Where there is a low amount of potassium (a mineral) in the blood caused by another diuretic (fluid-removing medicine)
Aldactone improves the blood pressure lowering action of thiazide diuretics while at the same time reducing or preventing potassium loss due to these medicines. - For the prevention of low amounts of potassium in the blood in patients taking digitalis (a type of heart drug)
- For the treatment of hirsutism (excess body hair in women)
How Aldactone works
Aldactone acts by working against a hormone called aldosterone. Too much aldosterone causes increased amounts of sodium (a mineral) and water to be retained by the kidneys, while too much potassium is removed from the body. Aldactone works against the effects of aldosterone.
Aldactone acts by removing excess fluid and by lowering blood pressure. It may be given alone or with other diuretics (fluid-removing medicines). It improves the effectiveness of other medicines used to lower blood pressure.
Aldactone also has a moderate ability to act against male sex hormones (anti-androgenic effect). Because of this, Aldactone is effective in the treatment of female hirsutism (excess body hair). It reduces hair growth, thickness and hair colour. Increased urine flow is unlikely to be a problem when Aldactone is used to treat hirsutism. This is because aldosterone levels are not normally high in patients with hirsutism.
The safety of Aldactone for the treatment of hirsutism in women of child-bearing age has not been established by specific studies.
Your doctor may recommend combined use with oral contraceptives to provide both regular menstrual cycles and adequate contraception.
Your doctor, however, may prescribe Aldactone for another purpose.
Ask your doctor if you have any questions about why Aldactone has been prescribed for you.
There is no evidence that Aldactone is addictive.
Before you take Aldactone
When you must not take it
Do not use Aldactone if:
- you are pregnant or think you might be pregnant
Aldactone should not be used during pregnancy due to possible effects on the developing baby (fetus). - you are breast feeding
The drug may appear in the breast milk and be passed to the infant. - you are allergic to Aldactone or to any of the tablet ingredients listed at the end of this leaflet
If you have an allergic reaction you may get a skin rash, have difficulty in breathing, or become faint. - you have severe kidney disease or are not passing urine
- you have hyperkalaemia (high levels of potassium in the blood)
- you have Addison's disease (a condition where the adrenal glands do not work properly).
Do not take Aldactone with potassium supplements or potassium containing salt substitutes or with potassium sparing diuretics.
Do not use tablets after the expiry date printed on the pack.
The tablets may have no effect at all, or an entirely unexpected effect, if you use them after the expiry date.
Do not use Aldactone if the packaging shows signs of tampering. If the product has expired or is damaged, return it to your pharmacist for disposal.
Do not use Aldactone to treat any other complaints unless your doctor advises you to.
Before you start to take it
You must tell your doctor if:
- you are already taking medicines for high blood pressure
- you are allergic to any other medicines, or any foods, dyes or preservatives
- you are taking any other medicines, including medicines that you buy without a prescription from a pharmacy, supermarket or health food shop.
Some of the medicines that may affect Aldactone or be affected by Aldactone include:
- other medicines used to treat high blood pressure
- diuretics, which are fluid removing medicines also known as water tablets
- digoxin, a medicine used to treat heart conditions
- medicines to prevent blood clots.
- potassium supplements or potassium sparing diuretics
- dietary salt substitutes. Many of these contain potassium
- cholestyramine, a medicine used to lower cholesterol levels in the blood
- ammonium chloride, which is contained in some cough and cold medicines
- aspirin
- non-steroidal anti-inflammatory medicines (NSAIDS) or other medicines which are used to relieve pain, swelling and other symptoms of inflammation, including arthritis.
- regional or general anaesthetics.
These medicines may be affected by Aldactone or may affect how well it works. You may need to take different amounts of your medicine or you may need to take different medicines. Your doctor or pharmacist has a more complete list of medicines to avoid while taking Aldactone.
How to take Aldactone
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the box, ask your doctor or pharmacist for help.
Your doctor or pharmacist will tell you how many tablets you will need to take each day. This depends on your condition and whether or not you are taking any other medicines.
How much to take
Daily doses of Aldactone in adults can range from 25 mg to 400 mg. Depending on the dose and your condition, Aldactone may be taken once a day or divided into separate doses.
In the treatment of hirsutism (excess body hair) in females, your doctor may tell you to take Aldactone every day or in repeating cycles with a break in between.
Follow your doctor's instructions exactly regarding the dose you should take and how often you should take it.
Doses of Aldactone in children are measured according to body weight and will be calculated by your doctor.
If you forget to take it
If you forget to take Aldactone, do not try to make up for missed doses by taking more than one dose at a time.
If you have trouble remembering when to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or Poisons Information Centre (telephone 13 11 26) or go to Accident and Emergency at your nearest hospital if you think that you or anyone else may have taken too much Aldactone. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
Keep telephone numbers for these places handy.
Overdose may cause nausea and vomiting. Sometimes, drowsiness, mental confusion, rash, diarrhoea or dehydration may occur.
While you are taking Aldactone
Things you must do
Take Aldactone exactly as your doctor has prescribed.
Tell all doctors, dentists and pharmacists who are treating you that you are taking Aldactone. This is especially important if you are going to receive an anaesthetic agent while being treated with Aldactone.
If you are about to have any blood tests tell your doctor that you are taking Aldactone. Aldactone may interfere with the results of some tests.
Tell your doctor if you're taking other types of medicines to treat high blood pressure.
Tell your doctor if you're taking medicines to prevent blood clots
Tell your doctor if you become pregnant while you are taking Aldactone. If it is possible for you to become pregnant, you should use adequate contraception while you are taking Aldactone.
Examples of adequate contraception are oral contraceptives ("the Pill") or intra-uterine devices (IUDs).
Stop taking Aldactone if you become pregnant or you think you may be pregnant.
Go to your doctor regularly for a check-up.
Your doctor may do blood tests to check your sodium and potassium levels and see how your kidneys are working.
Your doctor may do the blood test weekly at the start of your treatment, monthly for the first 3 months of treatment then quarterly for a year, and then every 6 months when increasing your dose.
You may need to stop taking Aldactone if your blood is high in potassium or if your kidneys are not working properly.
Things you must not do
Do not take potassium supplements or use salt substitutes that contain potassium.
Do not consume a diet rich in potassium. Dried fruit, bananas and oranges are some foods that contain high amounts of potassium. Consuming some of these foods is usually safe but do not consume excessive amounts.
If you are taking Aldactone, too much potassium can cause serious problems, such as disturbances to the heart rhythm.
Do not drive or operate machinery until you know how Aldactone affects you. Aldactone may cause drowsiness or dizziness in some people and may affect alertness.
Do not give this medicine to anyone else even if their symptoms seem similar to yours.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Aldactone. Like other medicines, Aldactone can cause some side effects. If they occur, they are most likely to be minor and temporary. However, some may be serious and need medical attention.
Aldactone helps most people with essential hypertension, oedematous disorders, primary aldosteronism, malignant hypertension and hirsutism but it may have unwanted side effects in a few people.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Ask your doctor or pharmacist to answer any questions you may have.
Following is a list of possible side effects. Do not be alarmed by this list. You may not experience any of them.
Tell your doctor if...
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- cramping or diarrhoea
- nausea or vomiting
- drowsiness, lethargy or generally feeling unwell
- skin rash or itchiness
- peeling skin or skin redness
- fever or sore throat
- unusual hair loss or thinning
- excessive hair growth
Tell your doctor as soon as possible if...
Tell your doctor as soon as possible if you notice any of the following:
- frequent infection such as fever, severe chills, sore throat or mouth ulcers. A few cases of agranulocytosis (lack of white blood cells) have been reported in patients taking Aldactone.
- breast enlargement. Breast enlargement may occur in men taking Aldactone. This normally goes away when Aldactone is stopped. In rare instances some breast enlargement may persist.
- breast lumps. Breast lumps and breast cancer have been reported in patients taking Aldactone although Aldactone has not been shown to cause breast cancer.
Go to hospital if...
If any of the following happen, stop taking Aldactone and either tell your doctor immediately or go to Accident and Emergency at your nearest hospital:
- stomach bleeding, ulcers or gastritis (inflammation of the stomach)
- unsteadiness when walking
- leg cramps
- headache
- mental confusion or dizziness
- change in sex drive
- impotence (inability to achieve or maintain an erection)
- breast pain
- irregular periods or no periods
- post-menopausal bleeding
- shortness of breath and swelling of the legs from fluid build (may be due to hyperkalaemia that is very serious)
These are very serious side effects. You may need urgent medical attention or hospitalisation.
Other side effects not listed above may also occur in some people.
After taking Aldactone
Storage
Keep your tablets in their original packaging, including outer carton, until it is time to take them.
If you take the medicine out of the pack it may not keep well.
Keep Aldactone in a cool dry place where the temperature stays below 30°C.
Do not store Aldactone or any other medicines in a bathroom or near a sink. Do not leave it in the car or on window sills.
Heat and dampness can destroy some medicines.
Keep Aldactone where children cannot reach it.
A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking Aldactone, ask your pharmacist what to do with any tablets left over.
Product description
What Aldactone looks like
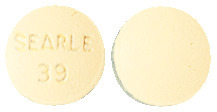
Aldactone 25 mg tablets are round, biconvex, buff coloured, peppermint flavoured, film coated, stamped SEARLE over 39 on one side and unmarked on the other side. The tablets are available in blister packs of 100 tablets.
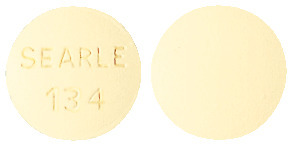
Aldactone 100 mg tablets are round, biconvex, buff coloured, peppermint flavoured, film coated, stamped SEARLE over 134 on one side and unmarked on the other side. The tablets are available in blister packs of 100 tablets.
Identification
Aldactone tablets can be identified by the Australian Registration number that appears on the carton.
25 mg tablets: AUST R 68953.
100 mg tablets: AUST R 68954.
Ingredients
The active ingredient in Aldactone tablets is spironolactone. Aldactone 25 mg tablets contain 25 mg spironolactone and Aldactone 100 mg tablets contain 100 mg spironolactone.
The tablets also contain:
- calcium sulfate
- maize starch
- povidone
- magnesium stearate
- hypromellose
- macrogol 400
- peppermint flavour
- Opaspray yellow
Supplier
Aldactone is supplied in Australia by:
Pfizer Australia Pty Ltd
Sydney NSW 2000
Toll free number: 1800 675 229
Date of preparation
This leaflet was prepared in February 2020
® Registered Trademark
© Pfizer Australia Pty Ltd 2020
Published by MIMS May 2020