What is in this leaflet
Read this leaflet carefully before taking your medicine.
This leaflet answers some of the common questions about Alendronate Plus D3 Sandoz. It is particularly important that you read the sections "When to take it" and "How to take it" before you take this medicine.
It does not contain all the information that is known about alendronate and colecalciferol.
It does not take the place of talking to your doctor and pharmacist.
All medicines have risks and benefits. Your doctor will have weighed the risks of you taking this medicine against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What this medicine is used for
The name of your medicine is Alendronate Plus D3 Sandoz. It contains the active ingredients alendronate sodium and colecalciferol.
It is used to treat osteoporosis and to provide additional vitamin D.
Osteoporosis is caused by changes in the way bone is normally maintained. Vitamin D (colecalciferol) is an essential nutrient required for calcium absorption and healthy bones.
Understanding bone
Bone is living, growing tissue. Throughout life, our bodies are breaking down old bone and rebuilding new bone in a continuous cycle. Until our late 20s, while bones are still developing, we gain bone by building more than we lose. From then until about age 35 the process is usually in balance, so that the amount of bone lost is about equal to the amount that is replaced. After about age 35 this balance is disturbed, with bone loss occurring at a slightly faster rate than it can be replaced. In women, after menopause, hormonal changes cause bone loss at an even faster rate. When bone loss is excessive, bones can become thinner and weaker, and therefore are more likely to break.
Osteoporosis
"Osteo" means bone, and "porosis" means something that has holes in it, like a sponge. Therefore, osteoporosis is a disease which causes bones to become more porous, gradually making them weaker, more brittle and likely to break.
Osteoporosis is common in postmenopausal women. The menopause occurs when the ovaries virtually stop producing the female hormone, oestrogen, or are removed (which may occur, for example, at the time of a hysterectomy). At this time, bone is removed faster than it is formed, so bone loss occurs and bones become weaker. The earlier a woman reaches the menopause, the greater the risk of osteoporosis.
Osteoporosis also occurs in men but is less common than in women.
Early on, osteoporosis usually has no symptoms. However, if left untreated it can result in broken bones, also called fractures. Although fractures usually cause pain, fractures of the bones of the spine may go unnoticed until they cause height loss. Fractures may occur during normal, everyday activity, such as lifting, or from minor injury that would not ordinarily fracture normal bone. Fractures usually occur at the hip, spine, or wrist and can lead not only to pain, but also to considerable deformity and disability, such as stooped posture from curvature of the spine, and loss of mobility.
What should I know about vitamin D?
Vitamin D is an essential nutrient, required for calcium absorption and healthy bones. The main source is through exposure to summer sunlight, which makes vitamin D in our skin. Clothing or sun block can prevent enough sunlight from getting through. In addition, as people age, their skin becomes less able to make vitamin D. Very few foods are natural sources of vitamin D.
Too little vitamin D leads to inadequate calcium absorption and low phosphate-minerals that make bones strong. Even if you are eating a diet rich in calcium or taking a calcium supplement, your body cannot absorb calcium properly unless you have enough vitamin D. Too little vitamin D may lead to bone loss and osteoporosis. Severe vitamin D deficiency may cause muscle weakness which can lead to falls and a higher risk of fracture.
How it works
Alendronate works by slowing down the process of old bone being removed, which allows the bone-forming cells time to rebuild normal bone. Alendronate not only helps prevent the loss of bone but actually helps to rebuild bone and make bone less likely to fracture.
Thus, alendronate reverses the progression of osteoporosis.
Alendronate starts working on the bone cells immediately, but measurable effects on bone mass may not be seen for several months or more.
Alendronate belongs to a group of non-hormonal medicines called bisphosphonates.
In addition to alendronate, your medicine also contains vitamin D, an essential nutrient required for calcium absorption and healthy bones.
Your doctor will have explained why you are being treated with this medicine.
Follow all directions given to you by your doctor carefully. They may differ from the information contained in this leaflet.
Your doctor may prescribe this medicine for another use. Ask your doctor if you want more information.
Alendronate Plus D3 Sandoz is not addictive.
Use in children
Do not give Alendronate Plus D3 Sandoz to a child as its effects in children have not been established.
Before you take this medicine
You should know that in some people, Alendronate Plus D3 Sandoz can irritate or burn the food pipe (also called oesophagus).
The chances of this happening should be reduced when you follow the instructions for 'How to take this medicine' in this leaflet.
When you must not take it
Do not take this medicine if:
- you have an allergy to alendronate, colecalciferol or any of the ingredients listed at the end of this leaflet
- you have certain disorders of the food pipe (oesophagus) including those that cause difficulty in swallowing
- you are unable to stand or sit upright for at least 30 minutes
- your doctor has told you that you currently have low blood calcium
- your dentist advises you to consult your doctor first
Do not take this medicine if you are pregnant or breast-feeding. Alendronate Plus D3 Sandoz has not been studied in pregnant or breast-feeding women.
Do not use after the use by (expiry) date printed on the pack. It may have no effect at all, or worse, an entirely unexpected effect if you take it after the expiry date.
Do not use this medicine if the packaging is torn or shows signs of tampering.
Do not use it to treat any other complaints unless your doctor tells you to.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Do not give this medicine to anyone else.
Before you start to take it
Tell your doctor if:
- You plan to become pregnant or breast-feed
- You have any medical conditions, especially the following:
- kidney disease
- swallowing or digestive problems, such as ulcers
- You have any allergies to any other medicines or any other substances, such as foods, preservatives or dyes
- You have dental or jaw-bone problems or are planning to have a course of dental surgery.
- You currently smoke or have been a smoker in the past.
If you have not told your doctor about any of the above, tell them before you take this medicine.
Taking other medicines
Tell your doctor if you are taking any other medicines including medicines that you buy at the chemist, supermarket or health food shop, including herbal medicines. Some medicines may affect the way other medicines work.
Some medicines are likely to interfere with the absorption of Alendronate Plus D3 Sandoz if taken at the same time. These include:
- antacids, medicines used to treat indigestion e.g. Gaviscon, Mylanta
- calcium supplements
- vitamins
Therefore, take this medicine at least 30 minutes before taking any of these or other medicines to make sure there is no problem with absorption. Check with your doctor or pharmacist if you are not sure whether you are taking any of these medicines.
You can take aspirin while you are being treated with this medicine.
However, both aspirin and Alendronate Plus D3 Sandoz may increase the chance of stomach upsets.
Your doctor or pharmacist has more information on medicines to be careful with or avoid while taking Alendronate Plus D3 Sandoz.
How to take this medicine
Follow carefully all directions given to you by your doctor or pharmacist. Their instructions may be different to the information in this leaflet.
How much to take
Take this medicine only when prescribed by your doctor.
The usual dose is one tablet once a week.
Choose the day of the week that best fits your schedule. Every week, take one tablet on your chosen day.
If you do not understand the instructions on the box, ask your doctor or pharmacist for help.
Do not increase or adjust your dose yourself.
When and how to take it
Take Alendronate Plus D3 Sandoz after getting up for the day. Do not take it at bedtime. Swallow one tablet whole with a full glass of plain water.
Do not take any food, medicines or drinks other than plain tap water with your tablet. It is important to take this medicine with plain water only, not mineral water. Food, other drugs and mineral water and other drinks, including fruit juices, coffee and tea, will reduce the effect of this medicine by interfering with the absorption into the body.
Stay upright for at least 30 minutes after swallowing the tablet and do not take any food, medicines or drinks other than plain tap water during this time.
Do not lie down immediately after swallowing it. It is important to stay upright (sitting, standing or walking around) for at least 30 minutes after swallowing your tablet.
It is also very important to stay upright until after you have eaten your first food of the day. These actions will help make sure your tablet reaches your stomach quickly and help reduce the potential for irritation to your food pipe (oesophagus).
Alendronate Plus D3 Sandoz is effective only if taken when your stomach is empty.
Food, drinks other than plain water, and other medicines will lessen the effect of this medicine by interfering with its absorption into the body.
Do not chew or suck on the tablet. Mouth ulcers may occur if the tablet is chewed or dissolved in the mouth.
How long to take it for
Continue taking your medicine for as long as your doctor tells you. Alendronate Plus D3 Sandoz can only treat your osteoporosis, by helping prevent further loss of bone and continuing to rebuild bone, if you take it every week.
Do not stop taking it unless your doctor tells you to - even if you feel better.
Make sure you have enough to last over weekends and holidays.
If you forget to take it
If you miss a tablet, take one tablet on the morning after you remember.
Do not take two tablets on the same day. Return to taking one tablet once a week, as originally scheduled on your chosen day.
If you are not sure about what to do, talk to your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints to help you remember.
If you take too much (overdose)
If you think that you or anyone else may have taken too much of this medicine, immediately telephone your doctor or the Poisons Information Centre (Tel: 13 11 26 in Australia) for advice. Alternatively go to the Accident and Emergency department at your nearest hospital.
Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
If you take too many tablets at one time, drink a full glass of milk. Do not induce vomiting. Do not lie down.
While you are taking this medicine
Things you must do
If you develop difficulty or pain upon swallowing, chest pain, or new or worsening heartburn, stop taking this medicine and call your doctor.
If you become pregnant while taking this medicine, stop taking the tablets and tell your doctor. Tell any other doctors, dentists and pharmacists who are treating you that you take this medicine.
If you develop a toothache or require a dental procedure, tell your dentist that you are taking this medicine.
If you develop new or unusual pain in your leg, tell your doctor. Rarely, patients have experienced fracture in a specific part of the thigh bone.
Make sure you have an adequate intake of calcium in your diet. Your doctor, dietician or pharmacist can tell you what foods you should eat.
Things you must not do
Do not:
- Stop taking your medicine, or change the dosage, without first discussing it with your doctor.
- Give this medicine to anyone else, even if their symptoms seem similar to yours.
- Take your medicine to treat any other condition unless your doctor or pharmacist tells you to.
Things to be careful of
There have been side effects reported with Alendronate Plus D3 Sandoz that may affect your ability to drive or operate machinery. Individual responses to this medicine may vary (see Side effects).
Things that would be helpful for your osteoporosis
Some self help measures suggested below may help your osteoporosis. Talk to your doctor or pharmacist about these measures and for more information.
- Exercise - can be helpful in building and maintaining strong bones. Regular exercise such as a brisk walk is a good idea. Talk to your doctor before you begin any exercise program.
- Diet - eat a balanced diet. You may need to increase the amount of calcium in your diet by eating calcium-rich foods or taking a calcium supplement. Your doctor will advise you.
- Smoking - appears to increase the rate at which you lose bone and, therefore, may increase your risk of fracture. Your doctor may ask you to stop smoking or at least cut down.
- Alcohol - your doctor may advise you to cut down the amount of alcohol you drink. If you drink excessively on a regular basis, you may increase your risk of developing osteoporosis.
Possible side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking this medicine or if you have any questions or concerns.
Alendronate Plus D3 Sandoz helps most people with osteoporosis, but it may have unwanted side effects in a few people. All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following:
- stomach pain, gas in the stomach or bowel, wind
- an uncomfortable feeling in the stomach or belching after eating, also called dyspepsia, or heartburn
- feeling sick (nausea), vomiting
- constipation, diarrhoea
- headache
- aching muscles, joints and/or bones, which rarely can be severe.
- flu-like symptoms typically at the start of treatment, such as aching muscles, generally feeling unwell and rarely fever.
- swelling of joints
- dizziness or spinning sensation
- unusual tiredness or weakness
- swelling of hands, ankles or feet
- hair loss
- changed sense of taste
Most of these are the more common side effects. For the most part, these have been mild.
Tell your doctor immediately if you notice any of the following:
- skin rash or redness of the skin, sometimes made worse by sunlight, itchiness
- mouth ulcers
- blurred vision, pain or redness in the eye
- symptoms of low blood calcium levels including muscle cramps or spasms or tingling sensation in the fingers or around the mouth
- new or unusual pain in your hip or thigh
These side effects are rare, and very rarely, may be serious.
Tell your dentist and doctor immediately if you notice any of the following:
- Jaw-bone or dental problems (including toothache). Jaw-bone problems may include infection, and delayed healing after a tooth extraction or other work that involves drilling into the jaw-bone.
These side effects are rare and may be serious.
If any of the following happen, stop taking this medicine and tell your doctor immediately:
- difficulty or pain upon swallowing
- chest pain
- new or worsening heartburn
These side effects may be due to irritation or ulceration of the food pipe. They may worsen if you continue taking the tablets. Rarely, these side effects may be serious.
Tell your doctor immediately or go to Accident and Emergency at your nearest hospital if you notice any of the following:
- swelling of the face, lips, mouth, throat or tongue which may cause difficulty in breathing or swallowing
- pinkish, itchy swellings on the skin, also called hives or nettlerash
- severe skin reactions
- black tar-like and/or bloody stools. These are all serious side effects. You may need urgent medical attention
These may be serious side effects. You may need urgent medical attention. These side effects are rare.
Tell your doctor if you notice anything else that is making you feel unwell. Other side effects not listed above may occur in some patients.
Do not be alarmed by this list of side effects. If you have the swelling described above, you may be having a serious allergic reaction to Alendronate Plus D3 Sandoz.
Rarely, stomach or duodenal ulcers (some severe) have occurred, but it is not known whether these were caused by this medicine.
Other side effects not listed above may also occur in some patients. Tell your doctor if you notice any other effects. Do not be alarmed by this list of possible side effects. You may not experience any of them.
Allergic reactions
If you think you are having an allergic reaction to Alendronate Plus D3 Sandoz, do not take any more of this medicine and tell your doctor immediately or go to the Accident and Emergency department at your nearest hospital.
Symptoms of an allergic reaction may include some or all of the following:
- cough, shortness of breath, wheezing or difficulty breathing
- swelling of the face, lips, tongue, or other parts of the body
- rash, itching or hives on the skin
- fainting
- hayfever-like symptoms
Storage and disposal
Storage
Keep your medicine in its original packaging until it is time to take it.
If you take your medicine out of its original packaging it may not keep well.
Keep your medicine in a cool dry place where the temperature will stay below 25°C.
Do not store your medicine, or any other medicine, in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep this medicine where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor or pharmacist tells you to stop taking this medicine or they have passed their expiry date, your pharmacist can dispose of the remaining medicine safely.
Product description
What Alendronate Plus D3 Sandoz looks like
Alendronate Plus D3 Sandoz 70 mg/70 mcg are white to off- white, modified capsule shaped uncoated tablet, debossed with 'ADC' on one side and '28' on the other side.
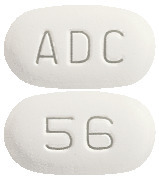
Alendronate Plus D3 Sandoz 70 mg/140 mcg are white to off- white, modified capsule shaped uncoated tablet, debossed with 'ADC' on one side and '56' on the other side.
Alendronate Plus D3 Sandoz are available in blister packs of 1 and 4 tablets.
Not all strengths and pack types may be available.
Ingredients
Each Alendronate Plus D3 Sandoz tablet contains 70 mg, of alendronate sodium and 70 mcg or 140 mcg of colecalciferol as the active ingredients.
It also contains the following inactive ingredients:
- microcrystalline cellulose
- medium chain triglycerides
- gelatin
- croscarmellose sodium
- sucrose
- silica-colloidal anhydrous
- magnesium stearate
- butylated hydroxytoluene
- povidone
This medicine is gluten-free, tartrazine-free and free of other azo dyes.
Australian Registration Numbers
Alendronate Plus D3 Sandoz 70 mg/70 mcg tablets: AUST R 206929
Alendronate Plus D3 Sandoz 70 mg/140 mcg tablets: AUST R 206935
Distributed by
Sandoz Pty Ltd
54 Waterloo Road,
Macquarie Park, NSW 2113
Australia
This leaflet was prepared in February 2015.
Published by MIMS April 2016


 In the two-year extension (treatment years 4 and 5) of the above studies, the overall safety profile of alendronate 10 mg/day was similar to that observed during the three-year placebo-controlled period. Additionally, the proportion of patients who discontinued alendronate 10 mg/day due to any clinical adverse experience was similar to that during the first three years of the study.
In the two-year extension (treatment years 4 and 5) of the above studies, the overall safety profile of alendronate 10 mg/day was similar to that observed during the three-year placebo-controlled period. Additionally, the proportion of patients who discontinued alendronate 10 mg/day due to any clinical adverse experience was similar to that during the first three years of the study.

 The effect of alendronate 70 mg/colecalciferol 70 microgram with an additional 70 microgram colecalciferol (2800 IU vitamin D3) for a total of 140 microgram colecalciferol (5600 IU vitamin D3) once weekly was compared to 70 mg/colecalciferol 70 microgram weekly in a 24-week, extension study that enrolled 652 osteoporotic men and post-menopausal women who completed the above 15-week study. Patients in the colecalciferol 70 microgram group received alendronate 70 mg/ colecalciferol 70 microgram (n=305 women, 21 men) and those in the colecalciferol 140 microgram group received alendronate 70 mg /colecalciferol 70 microgram with an additional 70 microgram colecalciferol (n=314 women, 12 men) once a week; additional vitamin D supplements were allowed. The primary endpoint was incidence of hypercalciuria, defined as an increase of greater than 25% from baseline in 24-hour urine calcium and to a value greater than the upper limit of normal (300 mg in women, 350 mg in men). The rate of hypercalciuria was 13/311 (4.2%) for the colecalciferol 140 microgram group and 9/317 (2.8%) for the colecalciferol 70 microgram group, relative risk 1.48 (95% CI 0.64, 3.40).
The effect of alendronate 70 mg/colecalciferol 70 microgram with an additional 70 microgram colecalciferol (2800 IU vitamin D3) for a total of 140 microgram colecalciferol (5600 IU vitamin D3) once weekly was compared to 70 mg/colecalciferol 70 microgram weekly in a 24-week, extension study that enrolled 652 osteoporotic men and post-menopausal women who completed the above 15-week study. Patients in the colecalciferol 70 microgram group received alendronate 70 mg/ colecalciferol 70 microgram (n=305 women, 21 men) and those in the colecalciferol 140 microgram group received alendronate 70 mg /colecalciferol 70 microgram with an additional 70 microgram colecalciferol (n=314 women, 12 men) once a week; additional vitamin D supplements were allowed. The primary endpoint was incidence of hypercalciuria, defined as an increase of greater than 25% from baseline in 24-hour urine calcium and to a value greater than the upper limit of normal (300 mg in women, 350 mg in men). The rate of hypercalciuria was 13/311 (4.2%) for the colecalciferol 140 microgram group and 9/317 (2.8%) for the colecalciferol 70 microgram group, relative risk 1.48 (95% CI 0.64, 3.40).


