What is in this leaflet
This leaflet answers some common questions about Allereze.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have benefits and risks. Your doctor or pharmacist may have advised you to take Allereze after weighing the risks against the benefits expected for you.
If you have any concerns about taking this medicine, talk to your doctor or pharmacist.
Keep this leaflet with your medicine. You may need to read it again.
What Allereze is used for
Allereze is a non-sedating antihistamine, used to relieve the symptoms of sneezing; runny nose; watery, itchy or red eyes in the following allergic conditions:
- hayfever (also known as seasonal allergic rhinitis), which usually occurs during the warmer seasons
- perennial allergic rhinitis, which may occur throughout the year.
Allereze can also be used to relieve the symptoms of hives or nettle rash (also known as chronic urticaria), which appears as a pinkish skin rash with itchy, swollen lumps.
The body releases histamine in response to substances it recognises as 'foreign', such as pollen, dust and dyes. This can cause the symptoms mentioned above. Antihistamines can relieve allergic symptoms by blocking the effects of histamine.
The severity of your condition will depend how sensitive you are to 'foreign substances'.
Ask your doctor or pharmacist if you have any questions about why Allereze has been recommended.
They may have recommended Allereze for another reason.
Allereze can be purchased without a prescription from pharmacies only.
There is no evidence that Allereze is addictive.
Before you take Allereze
When you must not take it
Do not take Allereze if you are allergic to any other medicines containing:
- loratadine (e.g. Claratyne, Clarinase, Lorastyne)
- desloratadine (e.g. Claramax)
- any of the ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include: skin rash, itching or hives; swelling of the face, lips or tongue which may cause difficulty in swallowing or breathing, wheezing or shortness of breath.
Do not give Allereze tablets to children under 12 years of age.
Do not take the tablets if they have passed the expiry date (EXP) printed on the pack. If you take this medicine after the expiry date, it may not work as well.
Do not take Allereze if the packaging shows signs of tampering or the tablets do not look quite right.
If you are not sure whether you should start taking this medicine, talk to your doctor or pharmacist.
Before you start to take it
Tell your doctor or pharmacist if you are pregnant or breastfeeding. Allereze is not recommended for use during pregnancy or while you are breastfeeding. Your doctor or pharmacist will discuss the risks involved.
Tell your doctor if you are allergic to any other medicines, foods, dyes or preservatives.
Tell your doctor or pharmacist if you have liver problems or any other medical conditions.
If you have not told your doctor about any of the above, tell them before you start taking Allereze.
Taking other medicines
Tell your doctor if you are taking any other medicines, including any that you buy without a prescription from a pharmacy, supermarket or health food shop.
How to take Allereze
Follow all directions given to you by your doctor and pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the pack, ask your doctor or pharmacist.
How much to take
Adults and children aged 12 years and older:
Take one tablet (10 mg) daily.
How to take Allereze
Swallow the tablet with a glass of water.
When to take Allereze
Allereze can be taken with or without food.
Allereze can be taken when the allergic symptoms start showing:
- hayfever may begin with an itchiness in the throat, nose or eyes
- hives will usually cause your skin to itch and you may notice pink lumps appearing.
People suffering from hayfever (seasonal allergic rhinitis) are more likely to experience symptoms during spring and summer, when there is more plant pollen in the air to trigger off symptoms.
How long to take Allereze for
You can stop taking Allereze when you obtain relief from the symptoms. It can be restarted if the symptoms recur.
If your condition does not improve or is not well controlled by Allereze, speak to your doctor or pharmacist.
If you take too much Allereze (overdose)
Immediately telephone your doctor, or the Poisons Information Centre (telephone 13 11 26), or go to Accident and Emergency at the nearest hospital, if you think you or anyone else may have taken too much Allereze. Do this even if there are no signs of discomfort or poisoning.
You may need urgent medical attention.
If you take too much Allereze, you may feel sleepy, have heart palpitations or a headache.
While you are taking Allereze
Things you must do
Tell all the doctors, dentists and pharmacists who are treating you that you are taking Allereze.
If you are planning to have skin tests to find out what you are allergic to, stop taking Allereze for 48 hours before the test. Allereze may interfere with the true results of the skin tests.
If you become pregnant while taking Allereze, tell your doctor.
If you already know which substances set off your allergies, keep a supply of Allereze ready so you can control the symptoms when they start appearing.
Things you must not do
Do not give Allereze to anyone else, even if they have similar symptoms to you. Other people who think Allereze may help their own condition should consider asking their doctor or pharmacist before taking Allereze for the first time.
Do not use Allereze to treat any other conditions unless your doctor or pharmacist tells you to.
Things to be careful of
Try to avoid contact with the substances you are allergic to.
Even though Allereze is a not likely to cause drowsiness you still need to be careful driving or operating machinery until you know how Allereze affects you.
If dizziness, lightheadedness or fainting occurs, do not drive, operate machinery or do anything else that could be dangerous.
These side effects may occur rarely in some people.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Allereze. Allereze helps most people with allergies. It is generally well tolerated but it may have unwanted side effects in some people.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the more common side effects listed below, and they worry you:
- headache
- feeling sleepy
- fatigue
- dry mouth.
If any of the following happen, stop taking it and tell your doctor immediately, or go to Accident and Emergency at the nearest hospital:
- sudden or severe signs of allergy such as skin rash, itching or hives
- swelling of the face, lips, tongue or other parts of the body, shortness of breath, wheezing or trouble breathing.
The above allergic side effects to Allereze are very rare and require urgent medical attention or hospitalisation.
Tell your doctor if you notice anything that is making you feel unwell. Other side effects not listed above may also occur in some patients.
After taking Allereze
Storage
Keep Allereze where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Keep your tablets in the blister pack until it is time to take them. If you take the tablets out of the pack they will not keep well.
Keep your tablets in a cool dry place where the temperature stays below 25°C.
Do not store Allereze or any other medicine in the bathroom or near a sink.
Do not leave Allereze in the car or on window sills. Heat and dampness can destroy some medicines.
Disposal
If your doctor or pharmacist tells you to stop taking Allereze, or your tablets have passed their expiry date, ask your pharmacist what to do with any that are left over.
Product description
What it looks like
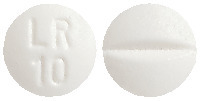
Allereze film-coated tablets are round, white, with a scoreline on one side and marked "LR" over "10" on the reverse, packs of 10, 30 and 50 tablets.
Ingredients
The active ingredient in Allereze is loratadine. Each Allereze tablet contains 10 mg of loratadine.
The tablets also contain the following inactive ingredients:
- lactose
- microcrystalline cellulose
- maize starch
- pregelatinised maize starch
- silicon dioxide
- magnesium stearate
- Opadry Clear YS-1R-7006
- carnauba wax
- purified talc.
Supplier
Allereze is supplied in Australia by:
Alphapharm Pty Limited
(ABN 93 002 359 739)
Chase Building 2
Wentworth Park Road
Glebe NSW 2037
Phone: (02) 9298 3999
www.alphapharm.com.au
Medical Information
Phone: 1800 028 365
Australian registration number:
Allereze - AUST R 117492
This leaflet was prepared on
9 March 2006.
Published by MIMS July 2006

 Molecular formula: C22H23N2ClO2. Molecular weight: 382.9.
Molecular formula: C22H23N2ClO2. Molecular weight: 382.9.