What is in this leaflet
Read this leaflet carefully before taking your medicine. This leaflet answers some common questions about candesartan cilexetil and hydrochlorothiazide (HCTZ). It does not contain all the available information.
It does not take the place of talking to your doctor or pharmacist.
The information in this leaflet was last updated on the date listed on the last page. More recent information on this medicine may be available.
Ask your doctor or pharmacist:
- if there is anything you do not understand in this leaflet,
- if you are worried about taking your medicine, or
- to obtain the most up-to-date information.
All medicines have risks and benefits. Your doctor has weighed the risks of you using this medicine against the benefits they expect it will have for you.
Pharmaceutical companies cannot give you medical advice or an individual diagnosis.
Keep this leaflet with your medicine. You may want to read it again.
What this medicine is used for
The name of your medicine is APO-Candesartan HCTZ tablets. It contains the active ingredients candesartan cilexetil and hydrochlorothiazide.
It is used to treat:
- high blood pressure, also called hypertension.
Ask your doctor if you have any questions about why this medicine has been prescribed for you.
They may differ from the information contained in this leaflet.
Your doctor may have prescribed this medicine for another reason.
This medicine is available only with a doctor's prescription.
How it works
Candesartan cilexetil is a type of medicine called an angiotensin II receptor inhibitor (or antagonist). It mainly works by causing relaxation of blood vessels.
Hydrochlorothiazide is a type of medicine called a diuretic. It works by reducing the amount of excess fluid in the body.
Using these two medicines together will lower your blood pressure more effectively than using either one on its own.
There is no evidence that this medicine is addictive.
Use in children
This medicine should not be used in children. The safety and effectiveness of this medicine in children under 18 years of age have not been established.
Before you use this medicine
When you must not use it
Do not use this medicine if:
- You have or have had any of the following:
- Allergic to any medicine containing candesartan cilexetil or hydrochlorothiazide, or any of the ingredients listed at the end of this leaflet, or any medicine containing an angiotensin II receptor antagonist (or blocker), or any sulphur drugs (sulphonamides) such as some antibiotics or some medicines to treat diabetes. Always check the ingredients to make sure you can use this medicine.
- severe kidney disease
- gout
- severe liver disease and/or conditions associated with impaired bile flow (cholestasis).
- taking blood pressure medicine containing aliskiren, especially if you have diabetes mellitus or kidney problems. - You are pregnant or planning to become pregnant.
This medicine may affect your developing baby if you take it during pregnancy. - You are breast-feeding or planning to breast-feed.
This medicine may pass into human breast milk. - You are hypersensitive to, or have had an allergic reaction to, candesartan cilexetil, hydrochlorothiazide or any of the ingredients listed at the end of this leaflet.
- You are hypersensitive to, or have had an allergic reaction to, any sulphur drugs (sulphonamides) such as antibiotics or some medicines used to treat diabetes.
- Any medicine containing an angiotensin II receptor antagonist (or blocker)
Symptoms of an allergic reaction may include cough, shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue, throat or other parts of the body; rash, itching or hives on the skin;
If you think you are having an allergic reaction, do not take any more of the medicine and contact your doctor immediately or go to the Accident and Emergency Department at the nearest hospital. - The expiry date (EXP) printed on the pack has passed.
- The packaging is torn, shows signs of tampering or it does not look quite right.
- If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Before you start taking this medicine, tell your doctor if:
- You have allergies to:
- any other medicines
- any other substances, such as foods, preservatives or dyes.
- You have or have had any medical conditions, especially the following:
- kidney problems
- liver problems
- heart problems
- diabetes
- recent excessive vomiting or diarrhoea
- Systemic Lupus Erythematosus (SLE), a disease affecting the skin, joints and kidneys
- a salt restricted diet
- a condition called primary hyperaldosteronism
- a past operation known as sympathectomy
- a decrease in vision or eye pain. These could be symptoms of fluid accumulation in the vascular layer of the eye (choroidal effusion) or an increase of pressure in your eye and can happen within hours to weeks of taking this medicine. This can lead to permanent vision loss, if not treated. If you earlier have had a penicillin or sulphonamide allergy, you can be at higher risk of developing this.
- experienced breathing or lungs problems (including inflammation or fluid in the lungs) following hydrochlorothiazide intake in the past.
- You are currently pregnant or you plan to become pregnant. Do not take this medicine whilst pregnant.
- You are currently breast-feeding or you plan to breast-feed. Do not take this medicine whilst breast-feeding.
- You have recently been vaccinated or plan to get a vaccination.
- You are planning to have surgery or an anaesthetic.
- You are currently receiving or are planning to receive dental treatment.
- You are taking or are planning to take any other medicines. This includes vitamins and supplements that are available from your pharmacy, supermarket or health food shop.
Some medicines may interact with Candesartan HCTZ.. These include:
- other medicines that lower blood pressure such as diuretics (fluid tablets) and ACE inhibitors, angiotensin II receptor blockers and aliskiren, especially if you have diabetes-related kidney problems
- medicines used to raise blood pressure.
- other medicines associated with potassium loss, such as other diuretics and laxatives.
- medicines containing potassium, including salt substitutes.
- Digoxin, a medicine used to treat heart failure
- other medicines used to treat irregular heartbeats.
- non-steroidal anti-inflammatory drugs (NSAIDs), medicines used to relieve pain, swelling and other symptoms of inflammation, including arthritis.
- Methotrexate, a medicine used to treat arthritis and some cancers
- colestipol and cholestyramine, medicines used to treat high blood cholesterol levels.
- lithium, a medicine used to treat mood swings and some types of depression.
- alcohol.
- strong pain killers such as codeine, morphine, dextropropoxyphene.
- barbiturates, used to treat epilepsy, such as phenobarbitone.
- medicines like insulin that are used to treat diabetes.
- calcium supplements, or medicines containing calcium.
- Vitamin D supplements
- Medicines to treat irregular heart beats
- corticosteroids such as prednisone, cortisone, dexamethasone.
- Laxatives
- amantadine, an antiviral and an antiparkinsonian medicine.
- cytotoxic medicines, such as medicines used for chemotherapy like cyclohoshamide.
- Methotrexate, a medicine used to treat arthritis and some cancers
- Ciclosporin, an immunosuppressant used to prevent organ transplant rejection.
If you are taking any of these you may need a different dose or you may need to take different medicines.
Other medicines not listed above may also interact with candesartan HCTZ. Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
How to take this medicine
Follow carefully all directions given to you by your doctor or pharmacist carefully.
Their instructions may be different to the information in this leaflet.
If you are not sure how to take this medicine, ask your doctor or pharmacist for help.
How much to take
Your doctor or pharmacist will tell you how much of this medicine you should take. This will depend on your condition and whether you are taking any other medicines.
Do not stop taking your medicine or change your dosage without first checking with your doctor.
The usual dose is one tablet once daily.
How to take it
Swallow the tablets whole with a glass of water.
When to take it
Take this medicine once a day, at about the same time each day. Taking it at the same time each day will have the best effect and will also help you remember when to take it.
It does not matter if you take it before, with or after food.
How long to take it
Continue taking this medicine for as long as your doctor tells you.
This medicine controls your condition, but does not cure it. Therefore you must take this medicine everyday. It is important to keep taking your medicine for as long as your doctor tells you to.
Make sure you have enough to last over weekends and holidays.
If you forget to take it
If you forget to take a dose, take it as soon as you remember, as long as it is at least 12 hours before your next dose is due.
Then go back to taking it as you would normally.
If it is less than 12 hours to your next dose, skip the dose you missed and take your next dose when you are meant to.
Do not take a double dose to make up for missed doses. This may increase the chance of you experiencing side effects.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints to help you remember.
If you take too much (overdose)
If you think that you or anyone else may have taken too much of this medicine, immediately telephone your doctor or the Poisons Information Centre (Tel: 13 11 26 in Australia) for advice. Alternatively go to the Accident and Emergency Department at your nearest hospital.
Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
If you take too many candesartan HCTZ tablets, you may get a headache, feel sick (nausea), dizzy, thirsty and very tired.
While you are taking this medicine
Things you must do
Tell your doctor that you are taking this medicine if:
- you are about to be started on any new medicine
- you are pregnant or are planning to become pregnant. Your doctor can discuss different treatment options with you.
- you are breast-feeding or are planning to breast-feed
- take this medicine exactly as your doctor has told you to. Your blood pressure will not be well controlled if you do not.
- you are about to have any blood tests
- you are going to have surgery or an anaesthetic or are going into hospital
- you experience excessive vomiting or diarrhoea.
You may lose too much water and your blood pressure may become too low. - if you plan to have an examination such as an X-ray or a scan requiring an injection of iodinated contrast (dye)
- If you have had skin cancer or if you develop a suspicious skin lesion during treatment with this medicine.
Treatment with hydrochlorothiazide, particularly long-term use with high doses, may increase the risk of some types of skin and lip cancer (nonmelanoma skin cancer). Limit exposure to sunlight and protect your skin when exposed to sun.
If you develop any severe shortness of breath or difficulty breathing after taking this medicine, seek medical attention immediately.
Your doctor may occasionally do tests to make sure the medicine is working and to prevent side effects. Go to your doctor regularly for a check-up.
Your doctor will check your progress and may want to take some tests (e.g. blood tests, blood pressure) from time to time. These tests may help to prevent side effects.
Tell any other doctors, dentists and pharmacists who are treating you that you take this medicine.
Things you must not do
Do not:
- Give this medicine to anyone else, even if their symptoms seem similar to yours.
- Take your medicine to treat any other condition unless your doctor tells you to.
- Stop taking your medicine, or change the dosage, without first checking with your doctor.
Things to be careful of
Be careful driving or operating machinery until you know how candesartan HCTZ affects you.
You may feel dizzy when you start taking candesartan HCTZ due to the drop in your blood pressure.
Move slowly when getting out of bed or standing up if you feel faint, dizzy or light-headed.
Drink plenty of water while you are using this medicine, especially if you sweat a lot.
Please talk to your doctor or pharmacist about these possibilities if you think they may be a problem for you.
Possible side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking this medicine or if you have any questions or concerns.
This medicine helps most people with high blood pressure, but it may have unwanted side effects in a few people.
Do not be alarmed by the following lists of side effects. You may not experience any of them. All medicines can have side effects. Sometimes they are serious but most of the time they are not. You may need medical treatment if you get some of the side effects.
Tell your doctor if you notice any of the following:
- headache or dizziness
- flu-like symptoms or infections
- chest, throat or sinus infections
- feeling sick (nausea) or vomiting
- back pain
- urinary tract infection
- feeling tired
- stomach ache
- Symptoms of sunburn which happens more quickly than normal.
These side effects are usually mild.
Tell your doctor as soon as possible if you notice any of the following:
- aching muscles, tenderness, or weakness in the muscle.
- Rapid heartbeats
- Suspicious skin lesions
The above list includes serious side effects and are usually rare. They may require medical attention.
If you experience any of the following, stop taking your medicine and contact your doctor immediately or go to Accident and Emergency at your nearest hospital:
- swelling of the face, lips, tongue or throat
- swelling of the hands, feet or ankles
- harsh sounds when breathing
- rash, itching or hives
- easy bruising or bleeding more easily than normal
- feeling extremely tired
- yellowing of the skin and/or eyes
- signs of frequent infections such as fever, severe chills, sore throat or mouth ulcers
- worsening of the kidney function including passing little or no urine, drowsiness, nausea, vomiting, breathlessness, loss of appetite and weakness (especially in patients with existing kidney problems or heart failure)
- changes in your potassium, sodium and red or white blood cell levels may occur. Such changes are usually detected by a blood test
- decrease in vision or pain in your eyes due to high pressure (possible signs of fluid accumulation in the vascular layer of the eye (choroidal effusion) or acute angle-closure glaucoma)
- symptoms that may indicate high potassium levels in the blood include nausea, diarrhoea, muscle weakness and changes in heart rhythm
- acute respiratory distress (very rare); signs include severe shortness of breath, fever, weakness and confusion.
These are very serious side effects. If you have them, you may have had a serious reaction to this medicine and are usually very rare. You may need urgent medical attention or hospitalisation.
Tell your doctor if you notice anything else that is making you feel unwell.
Other side effects not listed above may above may occur in some patients. Tell your doctor if you notice any other side effects.
Allergic reactions
If you think you are having an allergic reaction to candesartan HCTZ, do not take any more of this medicine and tell your doctor immediately or go to the Accident and Emergency department at your nearest hospital.
Symptoms of an allergic reaction may include some or all of the following:
- shortness of breath, wheezing or difficulty breathing
- swelling of the face, lips, tongue, or other parts of the body
- rash, itching or hives on the skin
Storage and disposal
Storage
Keep your tablets in the blister pack until it is time to take them. If you take candesartan HCTZ out of the blister pack it will not keep well.
Keep your tablets in a cool dry place where the temperature will stay below 25°C.
Do not store your medicine, or any other medicine, in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep this medicine where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor or pharmacist tells you to stop taking this medicine or it has passed its expiry date, your pharmacist can dispose of the remaining medicine safely.
Product description
What APO-Candesartan HCTZ looks like
APO-Candesartan HCTZ 16/12.5 mg tablets: light pink, oval shape, biconvex, uncoated, mottled tablet debossed with 'L3' and '02' on either side of breakline on one side and break line on other side
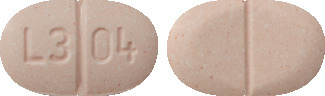
APO-Candesartan HCTZ 32/12.5 mg tablets: light yellow, oval, biconvex, uncoated, mottled tablet debossed with 'L3' & '04' on either side of breakline on one side and break line on other side
APO-Candesartan HCTZ 32/25 mg tablets: light pink, oval, biconvex, uncoated, mottled tablet debossed with 'L3' & '04' on either side of break line on one side & break line on one other side
Ingredients
APO-Candesartan HCTZ Tablets 16/12.5 mg contains 16 mg candesartan cilexetil and 12.5 mg of hydrochlorothiazide.
APO-Candesartan HCTZ Tablets 32/12.5 mg contains 32 mg candesartan cilexetil and 12.5 mg of hydrochlorothiazide.
APO-Candesartan HCTZ Tablets 32/25 mg contains 32 mg candesartan cilexetil and 25 mg of hydrochlorothiazide.
APO-Candesartan HCTZ is available in:
Blister packs of 7 and 30 tablets.
Not all strengths, and/or pack sizes may be available.
Ingredients
Each tablet contains 16 mg or 32mg of candesartan and 12.5mg or 25mg of HCTZ as the active ingredient.
It also contain the following inactive ingredients:
- lactose monohydrate
- carmellose calcium
- Maize starch
- macrogol 8000
- Hyprolose
- magnesium stearate
- Pigment Blend PB-24880 Pink (16/12.5 mg and 32/25 tablet only)
- (iron oxide yellow) (32/12.5 mg tablet only).
This medicine is gluten-free, sucrose-free, tartrazine-free and free of other azo dyes. The medicine contains sugars as lactose.
Australian Registration Numbers
APO-Candesartan HCTZ 16/12.5 mg blister pack: AUST R 210565
APO-Candesartan HCTZ 32/12.5 mg blister pack: AUST R 210566
APO-Candesartan HCTZ 32/25 mg blister pack: AUST R 210567
Sponsor
Arrotex Pharmaceuticals Pty Ltd
15-17 Chapel St
Cremorne 3121
VIC Australia
www.arrotex.com.au
This leaflet was prepared in May 2023.
Published by MIMS July 2023



 The following clinical adverse events occurred with a frequency of 0.5% to < 1% with no occurrence in the placebo group: A-V block, vomiting.
The following clinical adverse events occurred with a frequency of 0.5% to < 1% with no occurrence in the placebo group: A-V block, vomiting.
 Chemical Name: (±)-1-(cyclohexyloxycarbonyl-oxy) ethyl 2-ethoxy-1-[[2'-(1H-tetrazol-5-yl) biphenyl-4-yl] methyl]-1H-benzimadozole-7-carboxylate.
Chemical Name: (±)-1-(cyclohexyloxycarbonyl-oxy) ethyl 2-ethoxy-1-[[2'-(1H-tetrazol-5-yl) biphenyl-4-yl] methyl]-1H-benzimadozole-7-carboxylate. Chemical Name: 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulphonamide 1, 1-dioxide.
Chemical Name: 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulphonamide 1, 1-dioxide.