What is in this leaflet
This leaflet answers some common questions about lansoprazole. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking this medicine against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What this medicine is used for
The name of your medicine is APO-Lansoprazole. It contains the active ingredient lansoprazole.
In adults it is used to treat:
- reflux oesophagitis
- peptic ulcers
- Helicobacter pylori infection in patients with peptic ulcer or chronic gastritis
- reflux-like and/or ulcer-like symptoms associated with acid-related dyspepsia
In children aged 1-17 years of age it is used to treat:
- gastro-oesophageal reflux disease, including all grades of oesophagitis
- erosive oesophagitis
Lansoprazole belongs to a group of medicines called proton pump inhibitors (PPIs). It works by decreasing the amount of acid the stomach makes, to give relief from excessive acid and allow healing to take place.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed this medicine for another reason.
There is no evidence that this medicine is addictive.
This medicine is available only with a doctor's prescription.
Before you take this medicine
When you must not take it
Do not take this medicine if you have allergy to:
- lansoprazole or other proton pump inhibitors
- any of the ingredients listed at the end of this leaflet
Some of the symptoms of an allergic reaction may include:
- shortness of breath, cough, wheezing or difficulty breathing
- swelling of the face, lips, tongue, throat or other parts of the body
- rash, itching or hives on the skin
Do not take this medicine if you have severe liver disease.
Do not take this medicine if you are already taking atazanavir, a medicine used to treat HIV. Atazanavir is used to treat HIV infection. If it is taken at the same time as lansoprazole, it won't be absorbed properly and will be less effective in treating HIV infection.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to use it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you have or have had any of the following medical conditions:
- kidney or liver problems
- inflammation of the bowel
- a tumour in the stomach region
- osteoporosis
- low magnesium levels
- fructose intolerance, glucose galactose malabsorption or sucrose-isomaltase insufficiency.
Do not take this medicine if you are pregnant or plan to become pregnant.
Do not take lansoprazole whilst pregnant until you and your doctor have discussed the risks and benefits involved.
It may affect your developing baby if you take it during pregnancy.
Do not take this medicine if you are breastfeeding or plan to breastfeed. Do not take lansoprazole whilst breastfeeding until you and your doctor have discussed the risks and benefits involved.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and lansoprazole may interfere with each other. These include:
- theophylline, used to treat asthma
- carbamazepine and phenytoin used to treat seizures (fits)
- warfarin, used to prevent blood clots
- oral contraceptives
- sucralfate (used to treat stomach ulcers) and antacids (used to treat heartburn)
Lansoprazole should be taken at least an hour prior to taking sucralfate or an antacid.
- ampicillin esters, used in some antibiotics
- ketoconazole, used to treat fungal infections
- iron preparations
- digoxin, used to treat heart conditions
- tacrolimus or mycophenolate used in transplant patients to reduce organ rejection
- methotrexate, used in rheumatoid arthritis and some cancers to control immune response
- atazanavir, nelfinavir and others that require an acidic pH to be effective to treat HIV
- fluvoxamine, used to treat depression and anxiety
These medicines may be affected by lansoprazole or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking lansoprazole.
How to take this medicine
Follow all directions given to you by your doctor or pharmacist carefully. Their instructions may be different to the information in this leaflet.
If you do not understand the instructions on the label, ask your doctor or pharmacist for help.
How much to take
Your doctor will tell you how much of this medicine you should take. This will depend on your condition and whether you are taking any other medicines.
Adults
The dose is usually 30 mg a day. The dose may vary from 15 mg to 30 mg a day depending on your condition.
Children (6 years or older)
For children between 6 to 11 years, the recommended dose depends on the weight of the child.
For children weighing 30 kg or less, the usual dose is 15 mg daily.
For children weighing over 30 kg, the usual dose is one 30 mg tablet daily.
For children between 12 to 17 years, the dose may vary from 15 mg to 30 mg a day depending on the condition.
How to take it
The capsule should be swallowed whole with plenty of water. Do not crush or chew.
If you have difficulty swallowing this medicine, the capsule can be opened and taken as follows:
- Sprinkle the intact granules on one tablespoon of apple sauce, strained pears, cottage cheese or yoghurt and swallow immediately
- or sprinkle the intact granules into a small volume of either apple juice, orange juice or tomato juice. Mix briefly and swallow immediately.
To ensure complete delivery of the dose, the glass should be rinsed with two or more volumes of juice and the contents swallowed immediately.
Do not use other foods or liquids to swallow the granules because they have not been tested for use with this medicine.
If you have a nasogastric tube in place, this medicine may be given by a doctor or nurse by mixing the intact granules from the capsule with 40 mL of apple juice and injecting the mixture through the tube into the stomach. The tube is then flushed with more apple juice to clear it.
When to take it
Take this medicine in the morning before food.
Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
How long to take it for
Continue taking your medicine for as long as your doctor tells you.
If you forget to take it
If it is almost time to take your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take it as soon as you remember, and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for the dose that you missed. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much of this medicine. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
While you are taking this medicine
Things you must do
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking this medicine.
Tell any other doctors, dentists, and pharmacists who treat you that you are taking this medicine.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking this medicine. It may affect other medicines used during surgery.
If you become pregnant or start to breastfeed while taking this medicine, tell your doctor immediately.
If you are about to have any blood tests, tell your doctor that you are taking this medicine. Lansoprazole may interfere with the results of some tests.
Keep all your doctor's appointments so that your progress can be checked. Your doctor may do some tests from time to time to make sure lansoprazole is working and to prevent unwanted side effects.
Things you must not do
Do not take this medicine to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop taking your medicine or lower the dosage without checking with your doctor. If you stop taking it suddenly, your condition may worsen, or you may have unwanted side effects.
Things to be careful of
Be careful when driving or operating machinery until you know how this medicine affects you.
Lansoprazole may cause dizziness in some people.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking lansoprazole.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if you notice any of the following:
Stomach or bowel problems such as:
- diarrhoea, constipation
- indigestion
- nausea (feeling sick), vomiting
- flatulence or wind
- abdominal or stomach pain
Tell your doctor if you suffer from severe persistent diarrhoea and/or vomiting when taking lansoprazole.
As natural acid in the stomach helps to kill bacteria, the lowering of acid by acid-reducing medicines such as lansoprazole may cause some people to get certain stomach infections.
Difficulty in thinking or working due to:
- headache
- fatigue (tiredness)
- dizziness
- generally feeling unwell
- joint or muscle aches or pain
- feeling depressed, confused or having hallucinations
Changes to your appearance such as:
- thinning hair
- skin rashes
- hives or itchy skin
- breast enlargement and impotence in men (with long-term use)
Signs of infection such as:
- cough, cold, sore throat or sinus
- dry or sore mouth or throat
- frequent and painful passing of urine
Changes in sight, hearing, taste or touch including:
- taste disturbances
- tingling or numbness of hands and feet
- blurred vision
- increased sensitivity to sunlight
Tell your doctor as soon as possible if you notice any of the following:
- pain or indigestion
- vomiting blood or food
- passing black (blood-stained) motions
The above list includes serious side effects that may require medical attention.
If any of the following happen, tell your doctor immediately or go to Accident and Emergency at your nearest hospital:
- pain in the kidney region
- bruising or bleeding more easily than usual, bleeding under the skin or red or purple flat pinhead spots under the skin
- severe blisters and bleeding in the lips, eyes, mouth, nose or genitals (Stevens-Johnson syndrome)
- sudden or severe hives or itchy skin which may be accompanied by fever
- frequent infections such as fever, severe chills, sore throat or mouth ulcers
- watery or severe diarrhoea with stomach and bowel problems
- yellowing of the skin or eyes, especially if accompanied by fever, fatigue, loss of appetite, dark coloured urine or light-coloured bowel movements
- red, itchy blistering spots, especially if it appears in areas of the skin that are exposed to the sun and is accompanied by joint pain
- swelling of the face, lips, tongue or throat, which may cause difficulty breathing
- swelling of hands, ankles or feet
- cramping of the muscles in your hands or feet
- irregular heartbeat
- fits or seizures
- hallucinations.
These are very serious side effects and you may need urgent medical attention or hospitalisation.
Other side effects not listed above may occur in some patients.
Storage and disposal
Storage
Keep your medicine in its original pack until it is time to take them. If you take your medicine out of the original pack, they may not keep well.
Keep your medicine in a cool, dry place where the temperature will stay below 25°C.
Do not store this medicine or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one and a half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What APO- Lansoprazole looks like
APO-Lansoprazole 15 mg
Yellow cap/yellow body, self-locked hard gelatin capsules of size '3' imprinted with 'L 15' on both cap and body, containing white to off-white pellets. AUST R 159350. AUST R 159346.
APO-Lansoprazole 30 mg
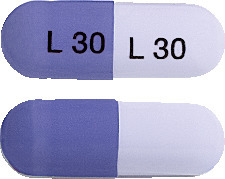
Purple cap/lavender body, self-locked hard gelatin capsules of size '1' imprinted with 'L 30' on both cap and body, containing white to off-white pellets. AUST R 159345. AUST R 159348
APO-Lansoprazole 15 mg and 30 mg are available in blister packs of 28 or 30 capsules.
Ingredients
Each APO-Lansoprazole Enteric Capsules contains 15 mg or 30 mg of lansoprazole as active ingredient.
It also contains the following inactive ingredients:
- sucrose
- maize starch
- purified talc
- hypromellose
- titanium dioxide
- methacrylic acid-ethyl acrylate copolymer (1:1)
- macrogol 300
- colloidal anhydrous silica
- gelatin
- Opacode S-1-277002 black
The 15 mg enteric capsule also contains the below colourants:
- iron oxide yellow CI77492
- quinoline yellow CI47005.
The 30 mg enteric capsule also contains the below colourants:
- indigo carmine CI73015
- carmoisine CI14720.
This medicine does not contain gluten and lactose. Contains sugars as sucrose. Contains sulfites.
Sponsor
Arrotex Pharmaceuticals Pty Ltd
15 – 17 Chapel Street
Cremorne VIC 3121
www.arrotex.com.au
This leaflet was last updated in:
September 2023.
Published by MIMS May 2025



 Treatment with lansoprazole also demonstrated significant reduction in frequency and severity of GORD symptoms (p < 0.001).
Treatment with lansoprazole also demonstrated significant reduction in frequency and severity of GORD symptoms (p < 0.001).
 The maintenance studies discussed, using lansoprazole 15 mg and 30 mg, did not extend beyond 12 months.
The maintenance studies discussed, using lansoprazole 15 mg and 30 mg, did not extend beyond 12 months. There was also a significant difference in the usage of "rescue" antacid treatment in the two groups, with 67% of the lansoprazole group taking antacids in the first two weeks of treatment compared with 83% of the ranitidine group (p = 0.001).
There was also a significant difference in the usage of "rescue" antacid treatment in the two groups, with 67% of the lansoprazole group taking antacids in the first two weeks of treatment compared with 83% of the ranitidine group (p = 0.001).
 Molecular formula: C16H14F3N3O2S.
Molecular formula: C16H14F3N3O2S.