What is in this leaflet
This leaflet answers some common questions about this medicine. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking this medicine against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What this medicine is used for
Levodopa/Carbidopa is used to treat some symptoms of Parkinson’s disease. This is a disease of the nervous system that mainly affects body movement. The three main symptoms are shaking (tremor), muscle stiffness, and slow and unsteady movement. People with Parkinson's disease often walk with a shuffle as they have difficulty in initiating movement. If untreated, Parkinson's disease can cause difficulty in performing normal daily activities.
Levodopa/Carbidopa is most helpful in improving slow movement and muscle stiffness. It is also frequently helpful in treating shaking, difficulty in swallowing and drooling.
The symptoms of Parkinson's disease are caused by a lack of dopamine, a naturally occurring chemical produced by certain brain cells. Dopamine relays messages in the part of the brain that controls muscle movement. When too little dopamine is produced, slowness of movement results.
APO-Levodopa/Carbidopa contains two active ingredients, levodopa and carbidopa. Levodopa is a chemical closely related to dopamine which allows the body to make its own dopamine. Carbidopa makes sure that enough levodopa gets to the brain where it is needed. In many patients, levodopa/carbidopa reduces some of the symptoms of Parkinson's disease.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
This medicine is available only with a doctor's prescription.
Before you take/use/give/are given this medicine
When you must not take it
Do not take this medicine if you have an allergy to:
- any medicine containing levodopa or carbidopa
- any of the ingredients listed at the end of this leaflet
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin
Do not take this medicine if you have any of the following medical conditions:
- any unusual skin lumps or moles which have not been examined by your doctor, or if you have ever had skin cancer or melanoma
- a type of glaucoma called narrow-angle glaucoma
Do not take this medicine if you are being treated for depression with certain medicines called Monoamine Oxidase Inhibitors (MAOIs) Ask your doctor or pharmacist if you are not sure whether you are taking one of these medicines.
Do not breastfeed if you are taking this medicine. Levodopa/Carbidopa passes into breast milk and there is a possibility that your baby may be affected.
Do not give this medicine to a child under the age of 18 years. Safety and effectiveness in children younger than 18 years have not been established.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you have or have had any of the following medical conditions:
- depression or mental disturbances
- heart disease, including irregular heartbeat (also known as arrhythmia)
- lung disease (including asthma)
- kidney, liver or hormonal problems
- convulsions or fits
- glaucoma
- peptic ulcer disease
Tell your doctor if you are pregnant or plan to become pregnant or are breastfeeding. Your doctor can discuss with you the risks and benefits involved.
Tell your doctor if you or your family member/caregiver notices that you are developing urges to gamble, excessive eating, spending or medicine use, repetitive purposeless activities with other medicines for Parkinson’s disease, and/or intense urges that could harm yourself or others. These behaviours are called impulse control disorders. Your doctor may need to review your treatments.
Tell your doctor if you have previously been or are currently being treated with levodopa.
If you have not told your doctor about any of the above, tell them before you start taking this medicine.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and levodopa/carbidopa may interfere with each other. These include:
- some medicines used to treat high blood pressure
- some medicines used to treat depression
- some medicines used to treat psychiatric problems
- some medicines used to treat diseases related to involuntary movements
- phenytoin, used to treat convulsions
- isoniazid, used to treat tuberculosis
- selegiline, another medicine used to treat Parkinson’s disease
- iron supplements and multivitamins containing iron
These medicines may be affected by levodopa/carbidopa or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
How to take this medicine
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the directions, ask your doctor or pharmacist for help.
How much to take
Your doctor will tell you how many tablets to take each day. The dose varies considerably from patient to patient.
The usual starting dose is one 100/25 mg tablet taken three times a day. Your doctor will then adjust this dose depending on the severity of your condition, your response to treatment and whether you are taking other medicines.
How to take it
Swallow the tablets with a full glass of water.
When to take it
Take your medicine at about the same time each day. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
How long to take it
Continue taking your medicine for as long as your doctor tells you.
This medicine helps to control your symptoms of Parkinson’s disease but does not cure it. It is important to keep taking your medicine even if you feel well.
If you forget to take it
If it is almost time to take your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take it as soon as you remember, and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for the dose that you missed. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much of this medicine. Do this even if there are no signs of discomfort or poisoning.
You may need urgent medical attention.
While you are using this medicine
Things you must do
You may feel light-headed or dizzy while taking this medicine. If you feel light-headed, dizzy or faint, get up slowly when getting out of bed or standing up. This is because your blood pressure is falling suddenly. Standing up slowly, especially when you get up from bed or chairs, will help your body get used to the change in position and blood pressure. If this problem continues or gets worse, tell your doctor.
Tell your doctor if you experience times where this medicine does not appear to be working as well as it did previously.
After taking this medicine for long periods of time, such as a year or more, some people suddenly lose the ability to move.
This loss of movement may last from a few minutes to several hours. The person is then able to move as before. This condition may unexpectedly occur again and again.
This problem is called the "on-off" effect. Your doctor may prescribe you a stronger dose of levodopa/carbidopa or may ask you to take it more frequently. Your doctor may need to prescribe you a different medicine.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking this medicine.
Tell any other doctors, dentists, and pharmacists who treat you that you are taking this medicine.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking this medicine. It may affect other medicines used during surgery.
If you become pregnant or start to breastfeed while taking this medicine, tell your doctor immediately.
If you are about to have any blood tests, tell your doctor that you are taking this medicine. It may interfere with the results of some tests.
Keep all your doctor's appointments so that your progress can be checked. Your doctor may do some tests from time to time (such as blood tests to check your blood, liver, kidneys or heart), to make sure the medicine is working and to prevent unwanted side effects.
Things you must not do
Do not take this medicine to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop taking your medicine or lower the dosage without checking with your doctor. If you stop taking it suddenly, your condition may worsen or you may have unwanted side effects such as muscle stiffness, fever or mental changes. If possible, your doctor will gradually reduce the amount you take each day before stopping the medicine completely.
Things to be careful of
Be careful driving or operating machinery until you know how this medicine affects you. This medicine may cause dizziness or light-headedness in some people. If you have any of these symptoms, do not drive, operate machinery or do anything else that could be dangerous. Children should be careful when riding bicycles or climbing trees.
In addition, in very rare cases, levodopa/carbidopa may cause excessive sleepiness and sudden onset of sleep. If you experience these effects, do not drive or operate machinery until these effects have resolved.
Be careful when drinking alcohol while you are taking this medicine. If you drink alcohol, dizziness or light-headedness may be worse.
If you feel light-headed, dizzy or faint when getting out of bed or standing up, get up slowly. Standing up slowly, especially when you get up from bed or chairs, will help your body to get used to the change in position and blood pressure. If this problem continues or gets worse, talk to your doctor.
Be careful not to eat a diet high in protein. The amount of levodopa absorbed by the body may be impaired if you eat a diet high in protein. Ask your doctor, pharmacist or dietician to check your diet.
If you are diabetic, check with your doctor or pharmacist before using urine sugar tests. Levodopa/Carbidopa may cause false results with some urine sugar tests.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking this medicine.
This medicine helps most people with their symptoms of Parkinson’s disease, but it may have unwanted side effects in a few people. All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
If you are over 65 years of age you may have an increased chance of getting side effects.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- abnormal uncontrolled movements including muscle twitching or spasms, which may or may not resemble your Parkinson's symptoms
- dizziness or light-headedness when standing quickly
- feeling sick (nausea), vomiting or loss of appetite
- discolouration of urine, sweat and/or saliva
- dream abnormalities
- sleepiness or sudden onset of sleep
- slow movements
- twitching or spasm of the eyelids
- hair loss
- diarrhoea
- tingling, numbness, pain or burning sensation in the feet and/or hands
Tell your doctor if you experience any of these behaviours. You may experience an inability to resist the impulse to perform an action that could be harmful, which may include:
- strong impulses to gamble
- increased sexual drive
- uncontrollable excessive shopping or spending
- bingeing or compulsive eating
- taking medicines and repetitive purposeless activities
- other urges
The above list includes the more common side effects of your medicine. They are usually mild and short-lived.
Tell your doctor as soon as possible if you notice any of the following:
- blood in the urine
- difficult or painful urination
- changes in mood, such as depression
- forgetfulness
- signs of anaemia, such as tiredness, being short of breath, and looking pale
- signs of frequent or worrying infections such as fever, severe chills, sore throat or mouth ulcers
- bruising or bleeding more easily than normal, or nose bleeds
- fainting
- skin rash or itchiness
- pinkish, itchy swellings on the skin, also called hives or nettle rash
- numbness or tingling in the hands or feet
- signs of melanoma, such as new skin spots or changes to the size, shape, colour or edges of an existing skin spot, freckle or mole
The above list includes serious side effects that may require medical attention. Serious side effects are rare.
If any of the following happen, tell your doctor immediately or go to Accident and Emergency at your nearest hospital:
- bleeding from the back passage, black sticky bowel motions (stools) or bloody diarrhoea
- vomiting blood or material that looks like coffee grounds
- chest pain
- fast or irregular heartbeats, also called palpitations
- muscle stiffness accompanied by fever
- mental changes such as feeling very fearful or paranoid, or hallucinations
- symptoms of an allergic reaction including cough, shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue, throat or other parts of the body; rash, itching or hives on the skin
The above list includes very serious side effects. You may need urgent medical attention or hospitalisation. These side effects are very rare.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Other side effects not listed above may also occur in some people.
Storage and Disposal
Storage
Keep your tablets in the bottle until it is time to take them. If you take the tablets out of the bottle they may not keep well.
Keep your medicine in a cool dry place where the temperature stays below 25°C.
Do not store this medicine or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
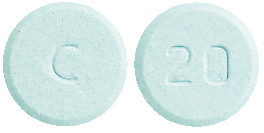
What APO-Levodopa/Carbidopa looks like
250/25
Round, light blue uncoated tablets with ‘C’ on one side and ‘20’ on the other side. AUST R 275984.
100/25
Round, light yellow uncoated tablets with ‘C’ on one side and ‘19’ on the other side. AUST R 275985.
The tablets are supplied in Alu/Alu blister packs of 100 tablets.
Ingredients
APO-Levodopa/Carbidopa 250/25 contains 250 mg of levodopa and 25 mg of carbidopa (as monohydrate).
APO- Levodopa/Carbidopa 100/25 contains 100 mg of levodopa and 25 mg of carbidopa (as monohydrate).
The tablets also contain the following inactive ingredients:
- crospovidone
- microcrystalline cellulose
- magnesium stearate
- pregelatinised maize starch
- indigo carmine aluminium lake (250/25 strength only)
- quinolone yellow aluminium lake (100/25 strength only)
Distributor
Arrotex Pharmaceuticals Pty Ltd
15 – 17 Chapel Street
Cremorne VIC 3121
Australia
Web: www.arrotex.com.au
This leaflet was updated in November 2022.
Published by MIMS January 2023