What is in this leaflet
This leaflet answers some common questions about this medicine. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you using this medicine against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with your medicine. You may want to read it again.
What this medicine is used for
The name of your medicine is APO-Perindopril Arginine/Amlodipine 5/5, 5/10, 10/5 & 10/10 tablets. It contains the active ingredient perindopril arginine and amlodipine besilate.
Perindopril belongs to a group of medicines called angiotensin converting enzyme (ACE) inhibitors.
Amlodipine belongs to a group of medicines called calcium channel blockers.
Calcium channel blockers do not change the amount of calcium in your blood or bones.
Perindopril Arginine/Amlodipine Tablets has been prescribed to you by your doctor to replace the separate tablets of perindopril and amlodipine you were taking.
One Perindopril Arginine/Amlodipine tablet replaces separate tablets of perindopril and amlodipine.
You have been prescribed Perindopril Arginine/Amlodipine tablets if you have high blood pressure, also known as hypertension.
Why Perindopril Arginine/Amlodipine is used for high blood pressure
Everyone has blood pressure. This pressure helps get your blood all around the body. Your blood pressure may be different at different times of the day, depending on how busy or stressed you are.
You have high blood pressure when your blood pressure stays higher than is needed, even when you are calm or relaxed.
There are usually no symptoms of high blood pressure. The only way of knowing that you have it is to have your blood pressure checked on a regular basis. If high blood pressure is not treated it can lead to serious health problems. You may feel fine and have no symptoms, but eventually it can cause stroke, heart disease and kidney failure.
The active ingredients perindopril arginine and amlodipine help lower your blood pressure.
You may also have been prescribed this medicine if you have coronary heart disease.
Why Perindopril Arginine/Amlodipine is used for coronary heart disease
Coronary heart disease is narrowing of the vessels carrying blood to the heart.
In patients with coronary artery disease, perindopril and amlodipine has been shown to reduce some of the risks, including heart attacks.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed this medicine for another reason.
This medicine is available only with a doctor's prescription.
How it works
Perindopril Arginine/Amlodipine tablets works by widening your blood vessels, which reduces pressure in the vessel, making it easier for your heart to pump blood around your body.
This helps increase the supply of oxygen to your heart, so that when you place extra demands on your heart, such as during exercise, your heart may cope better and you may not get short of breath as easily.
There is no evidence that this medicine is addictive.
Before you take this medicine
When you must not take it
Do not take this medicine if:
- You are pregnant or trying to become pregnant.
This medicine may affect your developing baby if you take it during pregnancy. - You are breastfeeding or plan to breastfeed.
This medicine passes into breast milk and therefore there is a possibility that the breast fed baby may be affected. - You are hypersensitive to, or have had an allergic reaction to, perindopril or amlodipine or any of the ingredients listed at the end of this leaflet.
- You have had an allergic reaction to any other ACE inhibitors or calcium channel blockers.
Symptoms of an allergic reaction may include: skin rash, itchiness, shortness of breath, swelling of the face, lips or tongue, muscle pain or tenderness or joint pain.
If you think you are having an allergic reaction, do not take any more of the medicine and contact your doctor immediately or go to the Accident and Emergency department at the nearest hospital. - You have experienced swelling of the face, tongue, lips or throat either spontaneously or in response to another medicine in the past. (This rare condition is known as angioedema).
- You are undergoing treatments where your blood is treated outside the body (also known as extracorporeal treatments) that may increase your risk of allergic reactions, treatments such as:
- renal dialysis or haemofiltration using polyacrylonitrile membranes.
- low-density lipoprotein (LDL) apheresis, a technique where LDL is ‘filtered’ out of the blood, using dextran sulfate. - You are treated with a blood pressure lowering medicine containing aliskiren and have diabetes or impaired kidney function.
- You are being treated with sacubitril/valsartan, a medicine for heart failure, as the risk of angioedema (rapid swelling under the skin in an area such as the throat) is increased (see also ‘Before you start to take it’ and ‘Taking other medicines’ sections).
- You have renal artery stenosis (narrowing of the blood vessels to one or both kidneys).
- You have aortic stenosis (narrowing of the main blood vessel leaving from the heart).
- You have severe hypotension (low blood pressure).
- You have unstable angina.
Unstable angina is a pain or uncomfortable feeling in the chest that lasts longer than a few minutes or occurs with rest, and may not be relieved with medication - You have had cardiogenic shock which is a sudden and severe drop in blood pressure and blood flow through the body because the heart is not pumping normally.
- You have had heart failure during the first 28 days after a heart attack (Heart failure means that the heart muscle cannot pump blood strongly enough to supply all the blood needed throughout the body. It does not mean that the heart stops working).
- The expiry date (EXP) printed on the pack has passed.
- The packaging is torn, shows signs of tampering or it does not look quite right.
For older people and patients with renal impairment
This medicine can generally be used safely by elderly people.
Reduced kidney function is often found in elderly people and in this case, the starting dose should always be 2.5mg of perindopril arginine and 2.5mg of amlodipine taken as separate tablets.
Use in children
This medicine should not be used in children.
Before you start to take it
Before you start taking this medicine, tell your doctor if:
- You have allergies to:
- any other medicines
- any other substances, such as foods, preservatives or dyes.
- You are currently pregnant or you plan to become pregnant. Do not take this medicine whilst pregnant as it may cause serious harm to your baby.
- You have or have had any medical conditions, especially the following:
- You are undergoing desensitisation treatment, or have had an allergic reaction during previous desensitisation treatment (e.g. treatments using bee, wasp or ant venom).
- you are undergoing, or you are intending to undergo, treatments where your blood is treated outside of the body (also known as extracorporeal treatments)
- you are to undergo anaesthesia and or surgery.
- You have recently suffered from diarrhoea or vomiting.
- You are on a salt restricted diet or use salt substitutes which contain potassium.
- You are treated with immunosuppressant therapy or allopurinol or procainamide.
- You have any other health problems, including:
- Kidney disease or if you undergo renal dialysis.
- Liver disease.
- High or low levels of potassium, or other problems with salt balance.
- Diabetes.
- Heart disease, severe increase in blood pressure or other heart problems.
- Systemic lupus erythematous or scleroderma (a disease affecting the skin, joints and kidneys).
- Abnormally increased levels of a hormone called aldosterone in your blood (primary hyperaldosteronism).
- You are taking any of the following medicines used to treat high blood pressure:
- An ‘angiotensin II receptor blocker’ (also known as ARBs or sartans - for example valsartan, telmisartan, irbesartan), in particular if you have diabetes-related kidney problems.
- Aliskiren.
- You are taking or are planning to take any other medicines. This includes vitamins and supplements that are available from your pharmacy, supermarket or health food shop.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interact with perindopril and/or amlodipine. These include:
- Some antibiotic medicines such as erythromycin, clarithromycin and rifampicin.
- Some anti-inflammatory medicines (including high dose aspirin, ibuprofen to relieve pain swelling and other symptoms of inflammation, including arthritis, and gold injections to treat rheumatoid arthritis).
- Medicines used to treat mood swings and some types of depression (lithium, tricyclic antidepressants, antipsychotics).
- Potassium-sparing medicines (spironolactone, triamterene, amiloride, eplerenone), sources of potassium, like potassium tablet and salt substitutes containing potassium, other medicines which increase potassium in your body (such as heparin, a medicine used to thin blood to prevent clots; co-trimoxazole also known as trimethoprim/ sulfamethoxazole for infections caused by bacteria; and ciclosporin, an immunosuppressant medicine used to prevent organ transplant rejection).
- Some medications used to treat high blood pressure (including angiotensin receptor blocker, beta-blockers, alpha-blockers), aliskiren or diuretics (sometimes called "fluid" or "water" tablets because they increase the amount of urine passed each day).
- Vasodilators including nitrates.
- Medicines used to treat diabetes (tablets and insulin).
- Muscle relaxants such as baclofen and dantrolene, dantrolene is also used to treat hyperthermia during anaesthesia (symptoms include very high fever and muscle stiffness).
- Medicines used to treat epilepsy such as carbamazepine, phenobarbitone, phenytoin or primidone.
- St. John’s Wort.
- Medicines which lower your immune system, such as corticosteroids, cyclosporin or medicines used to treat cancer (including radiation therapy).
- Simvastatin (cholesterol lowering medicine).
- Tetracosactide, a medicine used to treat adrenal insufficiency.
- Some medicines used to treat some fungal infections such as ketoconazole or itraconazole.
- Medicines which may affect the blood cells, such as allopurinol, procainamide.
- Medicines affecting the part of the nervous system that controls the activities of the heart and blood vessels, including ephedrine, noradrenaline or adrenaline.
- Alpha-blockers used for the treatment of enlarged prostrate such as prazosin, alfuzosin, doxazosin, tamsulosin and terazosin.
- Amifostine (used to prevent or reduce side effects caused by other medicines or radiation therapy that are used to treat cancer).
- Corticosteroids (used to treat various conditions including severe asthma and rheumatoid arthritis).
- Medicines used to treat HIV infection such as indinavir, ritonavir (also called ‘protease inhibitors’).
- Mammalian target of rapamycin (mTOR) inhibitors used to avoid rejection of transplanted organs (e.g. temsirolimus, sirolimus, everolimus)
- Sacubitril/valsartan (used to treat long term heart failure) (see also ‘Before you take this medicine’ and ‘Before you start to take it’sections).
- Gliptins used to treat diabetes (e.g. linagliptin, saxagliptin, sitagliptin, vildagliptin, alogliptin).
If you are taking any of these you may need a different dose or you may need to take different medicines.
Other medicines not listed above may also interact with perindopril and/or amlodipine.
How to take this medicine
Follow carefully all directions given to you by your doctor. Their instructions may be different to the information in this leaflet.
How much to take
Your doctor will tell you how much of this medicine you should take. The usual dose is one tablet once daily.
This will depend on your condition and whether you are taking any other medicines.
Do not stop taking your medicine or change your dosage without first checking with your doctor.
How to take it
Swallow your tablet with a glass of water.
Grapefruit juice and grapefruit should not be consumed by people who are taking this medicine. This is because grapefruit and grapefruit juice can lead to an increase in the blood levels of the active ingredient amlodipine, which can cause an unpredictable increase in the blood pressure lowering effect of this medicine.
When to take it
Take this medicine at the same time each day. Taking it at the same time each day will have the best effect and will also help you remember when to take it.
This medicine should be taken in the morning before a meal.
How long to take it for
Continue taking your medicine for as long as your doctor tells you.
Make sure you have enough to last over weekends and holidays.
If you forget to take it
If it is almost time to take your next dose, skip the missed dose and take your next dose at the usual time. Otherwise, take it as soon as you remember and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for missed doses. This may increase the chance of you experiencing side effects.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints to help you remember.
If you take too much (overdose)
If you think that you or anyone else may have taken too much of this medicine, immediately telephone your doctor or the Poisons Information Centre (Tel: 13 11 26 in Australia) for advice. Alternatively, go to the Accident and Emergency department at your nearest hospital.
Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
While you are taking this medicine
Things you must do
Tell your doctor that you are taking this medicine if:
- You are about to be started on any new medicine.
- You are pregnant or are planning to become pregnant.
- You are breastfeeding or are planning to breast-feed.
- You are about to have any blood tests.
- You are going to have surgery or an anaesthetic or are going into hospital.
Your doctor may occasionally do tests to make sure the medicine is working and to prevent side effects. Go to your doctor regularly for a check-up.
Tell any other doctors, dentists and pharmacists who are treating you that you take this medicine.
Things you must not do
Do not:
- Give this medicine to anyone else, even if their symptoms seem similar to yours.
- Take your medicine to treat any other condition unless your doctor tells you to.
- Stop taking your medicine, or change the dosage, without first checking with your doctor.
- Stop taking your tablets because you are feeling better, unless advised by your doctor.
Things that may help your condition
Some self-help measures suggested below may help your condition. Talk to your doctor or pharmacist about these measures and for more information.
- Alcohol - your doctor may advise you to limit your alcohol intake.
- Diet - eat a healthy low-fat diet which includes plenty of fresh vegetables, fruit, bread, cereals and fish. Also eat less fat and sugar.
- Exercise - regular exercise helps to reduce blood pressure and helps get the heart fitter, but it is important not to overdo it.
Walking is good exercise, but try to find a route that is reasonably flat. Before starting any exercise, ask your doctor about the best kind of program for you. - Salt - your doctor may advise you to watch the amount of salt in your diet. To reduce your salt in-take you should avoid using salt in cooking or at the table.
- Smoking - your doctor may advise you to stop or at least cut down smoking.
- Weight - your doctor may suggest losing some weight to help lower your blood pressure and help lessen the amount of work your heart has to do. Some people may need a dietician's help to lose weight.
Things to be careful of
This medicine may affect your ability to drive or use machines. If the tablets make you feel sick, dizzy, weak or tired, or give you a headache, do not drive or use machines and contact your doctor immediately.
Side effects
Tell your doctor as soon as possible if you do not feel well while you are taking Perindopril Arginine/Amlodipine tablets or if you have any questions or concerns.
Do not be alarmed by the following lists of side effects. You may not experience any of them. All medicines can have side effects. Sometimes they are serious but most of the time they are not.
Angioedema (a severe allergic reaction) has been reported in patients treated with ACE inhibitors, including perindopril arginine/amlodipine tablets. This may occur at any time during treatment. If you develop such symptoms described below, you should tell your doctor immediately or go to the Accident and Emergency department at your nearest hospital. These side effects are extremely rare but can become serious:
- Swelling of your extremities (limbs, hands or feet), lips, face, mouth, tongue or throat.
- Purple spots with occasional blisters on the front of your arms and legs and/or around your neck and ears (a rare condition known as Stevens-Johnson Syndrome, Toxic Epidermal Necrolysis (TEN)).
- Painful red areas, developing large blisters and peeling of layers of skin. This is accompanied by fever and chills.
- Red, often itchy spots, similar to the rash of measles, which starts on the limbs and sometimes on the face and the rest of the body.
- Difficulty in breathing.
- A fast and irregular heartbeat.
Talk to your doctor or pharmacist if you notice any of the following side effects.
Some of the side effects are usually identified after blood tests.
Very common (may affect more than 1 in 10 people) side effects can include:
- Dizziness.
- Cough, often described as dry and irritating, shortness of breath, discomfort on exertion.
- Oedema (fluid retention).
- Swelling of hands, ankles or feet, joint swelling (ankle swelling).
Common (may affect up to 1 in 10 people) side effects can include:
- Chest pain.
- Nose bleeds.
- Headache, vertigo, pins and needles.
- Changes in the rhythm or rate of the heartbeat, fast or irregular heartbeat.
- Feeling tired, lethargic or weak.
- Feeling sleepy (somnolence).
- Tinnitus (persistent noise in the ears), vision impairment (including double vision).
- Low blood pressure (and related effects), flushing, impaired peripheral circulation, blood vessel inflammation (vasculitis).
- Nausea, vomiting, taste disturbances, indigestion, diarrhoea, constipation, change of bowel habits, stomach pain or discomfort.
- Dry mouth.
- Muscle spasms.
- Fatigue.
- Rash, pruritus (itching), red raised skin rash.
- Eczema.
- Erectile dysfunction.
- Drowsiness.
- Chest tightness (dyspnoea), discomfort on exertion.
Uncommon (may affect up to 1 in 100 people) side effects can include:
- Conjunctivitis - discharge with itching of the eyes and crusty eyelids, swollen runny eyes.
- Runny or blocked nose, sneezing, facial pressure or pain.
- Bleeding or bruising more easily than normal caused by a low blood platelet count (thrombocytopenia).
- Frequent infections such as fever, severe chills, sore throat or mouth ulcers caused by lack of white blood cells (leukopenia).
- Hyperglycaemia (high blood sugar levels.
- Hypoglycaemia (low blood sugar levels).
- Thirst.
- Numbness or weakness of the arms and legs.
- Tremor.
- Numbness, or reduced sense of touch.
- High levels in the blood of potassium, urea and/or creatinine, low sodium levels in the blood.
- Peripheral ischaemia - a condition caused by reduced blood flow to the limbs, hands and feet.
- Orthostatic hypertension - dizziness on standing up, especially when getting up from a sitting or lying position.
- Mood disturbances, depression, nervousness, depersonalisation, sleep disturbances (difficulty sleeping, abnormal dreams), feeling sleepy or drowsy, fainting.
- Difficulty breathing or wheezing.
- Decreased appetite.
- Difficulty in swallowing.
- Flatulence or ‘passing wind’.
- Bleeding, tender or enlarged gums.
- Inflammation of the pancreas (pancreatitis).
- Swelling of the face, lips, mouth, tongue or throat which may cause difficulty in swallowing or breathing.
- Unusual hair loss or thinning.
- Purpura - unusual bleeding or bruising under the skin, or purple or red-brown spots visible through the skin.
- Excessive sweating.
- Increased sensitivity of the skin to sun, skin rash or inflammation of the skin often including blisters that weep and become crusted.
- Skin discolouration.
- Hypersensitivity reactions, mainly skin reactions, in patients with allergies and asthmatic reactions.
- Pemphigoid - a skin disease usually affecting older people.
- Increase in some white blood cells (eosinophilia).
- Pollakiuria.
- Need to urinate during the night.
- Passing urine more often than usual.
- Sexual dysfunction.
- Breast enlargement in men.
- Fever or high temperature.
- Kidney problems.
- Decreased blood sugar levels.
- Osteoarthritis.
- Aching muscles not caused by exercise.
- Back pain.
- Generally feeling unwell.
- Falls.
- Diplopia, eye pain.
- Fast, slow or irregular heartbeat.
- Peripheral coldness.
- Hives or skin rash (urticaria).
- Joint pain.
- Malaise, pain, chills.
- Blood urea/creatinine increase.
- Weight gain/weight decrease.
Rare (may affect up to 1 in 1,000 people) side effects can include:
- Increased appetite.
- Confusion, agitation.
- Apathy, lack of interest, enthusiasm, concern.
- Loss of memory.
- Ataxia - clumsiness and lack of coordination, affecting balance and manner of walking, limb or eye movements and/or speech. Unsteadiness when walking.
- Migraine.
- Inability to smell.
- Unusual muscle stiffness causing poor control of movement.
- Muscle weakness.
- Blurred vision.
- Dry eyes.
- Myocardial infarction, angina pectoris (a feeling of tightness, pressure or heaviness in the chest).
- Cardiac failure - disease of the heart with heart failure, symptoms include shortness of breath, swelling of the feet or legs due to fluid build-up.
- Gastritis - inflammation of the stomach where symptoms include pain, nausea, vomiting, vomiting blood, blood in the bowel motions.
- Red, often itchy spots, similar to the rash of measles, which starts on the limbs and sometimes on the face and the rest of the body.
- Dry skin.
- Cold and clammy skin.
- Dermatitis.
- Elevation of bilirubin levels in the blood, increases in liver enzymes.
- Hepatitis (liver disease).
- Yellowing of the skin and/or eyes, also called jaundice.
- Muscle twitching.
- Muscular weakness.
- Painful or difficult urination.
- Worsening of psoriasis.
- Acute kidney disease.
- Problems with production or passing of urine.
- Concentrated urine (dark in colour), feel or are sick, have muscle cramps, confusion and fits which may be due to inappropriate anti-diuretic hormone (ADH) secretion can occur with ACE inhibitors. If you have these symptoms contact your doctor as soon as possible.
Very rare (may affect up to 1 in 10,000 people) side effects can include:
- Hallucination.
- Cerebrovascular accident.
- Quicke’s oedema.
- Steven Johnson syndrome.
- Eosinophilic pneumonia.
- Illnesses resulting from a lack of destruction of red blood cells - (anaemia, pancytopenia).
- Illnesses resulting from a lack of white blood cells (agranulocytosis, neutropenia, pancytopenia).
- Severe flaking or peeling of the skin.
Not known (frequency cannot be estimated from the data available):
- Extrapyramidal syndrome - unusual movements, including trembling and shaking of the hands and fingers, rigid posture, mask-like face, slow movements of the body, shuffling unbalanced walk and stiffness of the arms and legs.
- Discolouration, numbness and pain in fingers or toes (Raynaud’s phenomenon).
Storage and disposal
Storage
Keep your medicine in its original packaging until it is time to take it. If you take your medicine out of its original packaging it may not keep well.
Keep your medicine in a cool dry place where the temperature will stay below 25°C.
Keep the container tightly closed and protect from moisture.
Do not store your medicine, or any other medicine, in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep this medicine where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or it has passed its expiry date, your pharmacist can dispose of the remaining medicine safely.
Product description
What APO-Perindopril Arginine/Amlodipine 5/5, 5/10, 10/5 & 10/10 Tablets looks like
5 mg/5 mg Tablet
White to off-white coloured, oval-shaped biconvex tablets engraved with "APO" on one side and "5/5"on the other side.
5 mg/10 mg Tablet
White to off-white coloured, square-shaped biconvex tablets engraved with "APO" on one side and "5/10" on the other side.
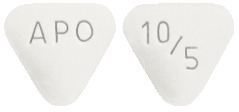
10 mg/5 mg Tablet
White to off-white coloured, triangular-shaped biconvex tablets engraved with "APO" on one side and "10/5" on the other side.
10 mg/10 mg Tablet
White to off-white coloured, round-shaped biconvex tablets engraved with "APO" on one side and "10/10" on the other side.
* Not all strengths, pack types and/or pack sizes may be available.
Ingredients
Each tablet contains perindopril arginine and amlodipine (as besilate) respectively: 5 mg/5 mg, 5 mg/10 mg, 10 mg/5 mg & 10 mg/10 mg as the active ingredients.
It also contains the following inactive ingredients:
- microcrystalline cellulose
- croscarmellose sodium
- colloidal silica anhydrous
- magnesium stearate
This medicine is gluten-free, lactose-free, sucrose-free, tartrazine-free and free of other azo dyes.
Australian Registration Numbers
APO-Perindopril Arginine/Amlodipine 5/5 Tablet
Bottle of 30 tablets:
AUST R 224306.
Blister pack of 30 tablets:
AUST R 224333.
APO-Perindopril Arginine/Amlodipine 5/10 Tablet
Bottle of 30 tablets:
AUST R 224292.
Blister pack (of 30 tablets:
AUST R 224325.
APO-Perindopril Arginine/Amlodipine 10/5 Tablet
Bottle of 30 tablets:
AUST R 224316.
Blister pack of 30 tablets:
AUST R 224309.
APO-Perindopril Arginine/Amlodipine 10/10 Tablet
Bottle of 30 tablets:
AUST R 224300.
Blister pack of 30 tablets:
AUST R 224312.
Sponsor
Arrotex Pharmaceuticals Pty Ltd
15-17 Chapel Street
Cremorne, VIC 3121
Australia
Web: www.arrotex.com.au
Tel:+61-1300927769
APO and APOTEX are registered trademarks of Apotex Inc.
This leaflet was last updated in:
August 2023.
Published by MIMS October 2023




 Food intake may reduce hepatic biotransformation of perindopril to perindoprilat. Recommended treatment is one tablet per day as a single dose, preferably to be taken in the morning and before a meal.
Food intake may reduce hepatic biotransformation of perindopril to perindoprilat. Recommended treatment is one tablet per day as a single dose, preferably to be taken in the morning and before a meal.

 Chemical Name: (2S,3aS,7aS)-1-[(2S)-2-[[(1S)-1-[Ethoxycarbonyl)butyl]amino] propanoyl]octahydro-1H-indole-2-carboxylic acid, L-arginine salt.
Chemical Name: (2S,3aS,7aS)-1-[(2S)-2-[[(1S)-1-[Ethoxycarbonyl)butyl]amino] propanoyl]octahydro-1H-indole-2-carboxylic acid, L-arginine salt. Chemical Name: 3-ethyl 5-methyl (4RS)-2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate benzene sulfonate.
Chemical Name: 3-ethyl 5-methyl (4RS)-2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate benzene sulfonate.