What is in this leaflet
Read this leaflet carefully before taking your medicine. This leaflet answers some common questions about quetiapine. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
The information in this leaflet was last updated on the date listed on the last page. More recent information on this medicine may be available.
Ask your doctor or pharmacist:
- if there is anything you do not understand in this leaflet,
- if you are worried about taking your medicine, or
- to obtain the most up-to-date information.
You can also download the most up to date leaflet from www.apotex.com.au.
All medicines have risks and benefits. Your doctor has weighed the risks of you using this medicine against the benefits they expect it will have for you.
Pharmaceutical companies cannot give you medical advice or an individual diagnosis.
Keep this leaflet with your medicine. You may want to read it again.
What this medicine is used for
The name of your medicine is APO-Quetiapine tablets. It contains the active ingredient quetiapine (as quetiapine fumarate). It is used to treat:
- the symptoms of schizophrenia, an illness which affects the way people think, feel and behave.
- Bipolar 1 disorder, an illness in which there are sustained mood swings, either up (mania) or down (depression). During mania, patients experience episodes of overactivity, elation or irritability. During depression, patients may feel depressed or guilty, lack energy, lose their appetite and have trouble sleeping. Quetiapine may both treat and prevent bipolar disorder from recurring.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed this medicine for another reason.
This medicine is available only with a doctor's prescription.
How it works
Quetiapine belongs to a group of medicines called antipsychotics.
It helps to correct chemical imbalances in the brain.
There is no evidence that this medicine is addictive.
Use in children
Do not give quetiapine to children or adolescents unless recommended by your doctor.
The effects of quetiapine have only been studied in children aged between 10 and 17 years with bipolar and in children aged between 13 and 17 years with schizophrenia. There is not enough information on its effects in children to recommend use in other age groups or for other conditions.
Before you take this medicine
When you must not take it
Do not take this medicine if:
-
You are hypersensitive to, or have had an allergic reaction to, quetiapine or any of the ingredients listed at the end of this leaflet.
Symptoms of an allergic reaction may include: cough, shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue, throat or other parts of the body; rash, itching or hives on the skin; fainting; or hayfever-like symptoms.
If you think you are having an allergic reaction, do not take any more of the medicine and contact your doctor immediately or go to the Accident and Emergency department at the nearest hospital. - The expiry date (EXP) printed on the pack has passed.
- The packaging is torn, shows signs of tampering or it does not look quite right.
Before you start to take it
Before you start taking this medicine, tell your doctor if:
- You have allergies to:
- any other medicines
- any other substances, such as foods, preservatives or dyes.
- You have or have had any medical conditions, especially the following:
- heart or blood vessel problems or a family history of heart or blood vessel problems, including: low blood pressure, stroke, problems with your circulation or any condition that affects blood flow to the brain, problems with the way your heart beats or a history of heart attack or prolonged QT interval.
- problems which may lead you to developing low blood pressure, e.g. being dehydrated, low blood volume, or taking certain blood pressure medicines
- low potassium or magnesium in your blood
- liver problems
- diabetes (or a family history of diabetes). Patients with diabetes or who have a higher chance of diabetes should have their blood sugar checked before and during treatment with this medicine.
- epilepsy (seizures or fits)
- dementia or related behavioural disorders (especially in elderly patients).
- low white blood cell count
- sleep apnoea, a sleep disorder that causes you to stop breathing for short periods during sleep.
- urinary retention, inability to empty the bladder
- benign prostatic hyperplasia (BPH), enlarged prostrate
- bowel obstruction or a blockage in your intestines
- glaucoma (increased pressure in your eyes)
- history of alcohol or drug abuse
- thoughts of suicide or self-harm. Depression and other mental illnesses can lead to suicide. It is important to discuss all the risks of treating depression and mental illness as well as the risks of not treating it. You should discuss all treatment choices with your doctor, not just the use of medicines. Patients (and caregivers of patients) need to monitor for any worsening of their condition and/or the emergence of thoughts of suicide or suicidal behaviour or thoughts of harming themselves and to seek medical advice immediately if these symptoms present.
- You are currently pregnant, or you plan to become pregnant.
Do not take this medicine whilst pregnant until you and your doctor have discussed the risks and benefits involved.
There have been some reports of side effects such as shaking, muscle stiffness, breathing difficulty and problems feeding in newborn babies whose mothers have taken quetiapine during the third trimester of pregnancy. - You are currently breastfeeding, or you plan to breastfeed.
Breast-feeding is generally not recommended when taking quetiapine. Do not take this medicine whilst breast-feeding until you and your doctor have discussed the risks and benefits involved.
Quetiapine may pass into human breast milk - You are planning to be in a situation where your body will be subject to extreme heat or you will be exercising strenuously, or likely to suffer from dehydration.
- You are having any blood or urine tests
- You are planning to have surgery or an anaesthetic.
- You are currently receiving or are planning to receive dental treatment.
- You are taking or are planning to take any other medicines. This includes vitamins and supplements that are available from your pharmacy, supermarket or health food shop.
Some medicines may interact with quetiapine. These include:
- medicines used to treat anxiety, depression, mood swings, attention deficit hyperactivity disorder (ADHD) or other mood disorders
- lorazepam, a medicine used to help you sleep
- certain medicines for treating epilepsy, such as phenytoin and carbamazepine
- medicines for high blood pressure (including diuretics or fluid tablets) or heart conditions
- some antibiotics such as rifampicin and erythromycin
- medicines used for fungal infections such as ketoconazole
- medicines for Human Immunodeficiency Virus (HIV)
- other antipsychotic medicines such as thioridazine
- medicines used to treat Parkinson's disease
- medicines used to treat urinary retention and benign prostatic hyperplasia (enlarged prostrate)
- medicines that have anti-cholinergic effects
- medicines used to treat glaucoma
- stimulants such as amphetamines
- glucocorticoids - steroid medicines used to treat inflammation.
These medicines may be affected by quetiapine or may affect how well it works. You may need a different dose, or you may need to take different medicines. Your doctor will advise you.
Other medicines not listed above may also interact with quetiapine.
How to take this medicine
Follow carefully all directions given to you by your doctor. Their instructions may be different to the information in this leaflet.
How much to take
Your doctor will tell you how much of this medicine you should take. This will depend on your condition and whether you are taking any other medicines.
Quetiapine fumarate is usually taken once or twice a day.
If you are elderly or if you have liver problems your doctor may prescribe a lower dose. Your doctor will monitor your condition and may change your dose depending on how you respond to it.
Do not stop taking your medicine or change your dosage without first checking with your doctor.
How to take it
Swallow your tablets whole with a full glass of water.
When to take it
Take this medicine at the same time each day. Taking it at the same time each day will have the best effect and will also help you remember when to take it.
It does not matter if you take it before, with or after food.
How long to take it
Continue taking your medicine for as long as your doctor tells you.
Quetiapine helps control your condition but does not cure it. Therefore, you must take it every day.
Do not stop taking it suddenly unless your doctor tells you to - even if you feel better.
Make sure you have enough to last over weekends and holidays.
If you forget to take it
If it is almost time to take your next dose, skip the missed dose and take your next dose at the usual time. Otherwise, take it as soon as you remember and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for missed doses. This may increase the chance of you experiencing side effects.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints to help you remember.
If you take too much (overdose)
If you think that you or anyone else may have taken too much of this medicine, immediately telephone your doctor or the Poisons Information Centre (Tel: 13 11 26 in Australia) for advice. Alternatively, go to the Accident and Emergency department at your nearest hospital.
Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
If you take too much quetiapine, you may feel drowsy, sleepy, and dizzy or have fast heart beats.
While you are taking this medicine
Things you must do
Tell your doctor that you are taking this medicine if:
- you are about to be started on any new medicine
- you are pregnant or are planning to become pregnant (tell your doctor immediately)
- you are breastfeeding or are planning to breast-feed
- you are about to have any blood or urine tests
- you are going to have surgery or an anaesthetic or are going into hospital.
Your doctor may occasionally do tests to make sure the medicine is working and to prevent side effects. Go to your doctor regularly for a check-up.
If you or someone you know is demonstrating any of the following warning signs of suicide while taking quetiapine, contact your doctor or a mental health professional immediately or go to the nearest hospital for treatment:
- Thoughts or talk of death or suicide.
- Thoughts or talk of self-harm or harm to others.
- Any recent attempts of self-harm
- Increase in aggressive behaviour, irritability or agitation.
- Worsening of depression.
Occasionally, the symptoms of depression may include thoughts of suicide or self-harm. These symptoms may continue or get worse during the early stages of treatment until the effect of the medicine becomes apparent.
All mentions of suicide or violence must be taken seriously.
Tell any other doctors, dentists and pharmacists who are treating you that you take this medicine.
Things you must not do
Do not:
- Give this medicine to anyone else, even if their symptoms seem similar to yours.
- Take your medicine to treat any other condition unless your doctor tells you to.
- Stop taking your medicine suddenly, or change the dosage, without first checking with your doctor. If your doctor decides that you need to stop taking quetiapine, then he/she will reduce the dose gradually over one to two weeks.
- Take any other medicines that cause drowsiness while you are taking quetiapine, unless recommended by a doctor.
Things to be careful of
Be careful when driving or operating machinery until you know how this medicine affects you, as it may impair judgement, thinking or reactions.
If quetiapine makes you feel light-headed, dizzy or faint, be careful when getting up from a sitting or lying position. Standing up slowly, especially when you get up from bed or chairs, will help your body get used to the change in position and blood pressure. If this problem continues or gets worse, talk to your doctor.
Be careful when drinking alcohol while you are taking quetiapine. Combining your medicine and alcohol can make you more sleepy or dizzy.
Your doctor may suggest you avoid alcohol while you are being treated with this medicine.
Make sure you keep cool in hot weather and warm in cool weather. Take care also if doing strenuous exercise and be certain to maintain your fluid levels. Quetiapine may affect the way your body reacts to temperature changes.
Avoid drinking large quantities of grapefruit juice. This medicine may be affected by grapefruit juice.
Possible side effects
Tell your doctor as soon as possible if you do not feel well while you are taking quetiapine or if you have any questions or concerns.
Do not be alarmed by the following lists of side effects. You may not experience any of them. All medicines can have side effects. Sometimes they are serious but most of the time they are not.
Tell your doctor if you notice any of the following and they worry you:
- weight gain, increased appetite
- feeling weak or lethargic
- dry mouth
- runny or stuffy nose
- headache
- nausea, vomiting, indigestion, upset stomach, constipation or diarrhoea
- swelling of your hands, feet or ankles
- blurred vision or other sight problems
- feeling sleepy or having trouble getting to sleep
- abnormal dreams, nightmares
- irritability
- fall in blood pressure, especially on standing. This will be apparent to you as light-headedness or dizziness that passes after a few seconds or after sitting down again.
Tell your doctor as soon as possible if you notice any of the following.
These may be serious side effects and you may need medical attention:
- shortness of breath, difficulty in breathing and/or tightness in the chest, or chest infection.
- low body temperature
- difficulty in speaking
- difficulty in swallowing
- passing only small amounts of urine
- fast, irregular heartbeats (palpitations)
- sleep walking or doing other activities whilst asleep
- symptoms of high sugar levels in the blood (including passing large amounts of urine, excessive thirst, increase in appetite with a loss of weight, feeling tired, drowsy, weak, depressed, irritable and generally unwell)
- breast enlargement, unusual secretion of breast milk.
If you experience any of the following, stop taking your medicine and contact your doctor immediately or go to the Accident and Emergency department at your nearest hospital.
These are very serious side effects and you may need urgent medical attention or hospitalisation:
- thoughts or actions regarding harming or killing oneself.
- long lasting and painful erection
- fainting or passing out
- signs of frequent infections such as fever, chills, sore throat or mouth ulcers
- bleeding or bruising more easily than normal
- very marked drowsiness
- reduced consciousness
- abnormal muscle movement, including an overwhelming urge to move your legs, difficulty starting muscle movements, shaking, restlessness or muscle stiffness without pain.
- worm-like movements of the tongue, or other uncontrolled movements of the tongue, mouth, cheeks or jaw which may progress to the arms and legs.
- a sudden increase in body temperature, with sweating, or a fast heart beat
- fits (seizures)
- severe upper stomach pain, often with nausea and vomiting (particularly in patients with other risk factors such as gallstones, alcohol consumption and/or increased levels of certain fats within the blood).
Occasionally, quetiapine may be associated with changes in your liver function or blood (e.g. blood fat levels such as cholesterol or triglycerides, blood sugar levels, thyroid hormone levels, white blood cells), which may require your doctor to do certain blood tests.
Other side effects not listed above may occur in some patients.
Allergic reactions
If you think you are having an allergic reaction to quetiapine, do not take any more of this medicine and tell your doctor immediately or go to the Accident and Emergency department at your nearest hospital.
Symptoms of an allergic reaction may include some or all of the following:
- cough, shortness of breath, wheezing or difficulty breathing
- swelling of the face, lips, tongue, throat or other parts of the body
- rash, itching or hives on the skin
- fainting
- hay fever-like symptoms.
Storage and disposal
Storage
Keep your medicine in its original packaging until it is time to take it.
If you take your medicine out of its original packaging it may not keep well.
Keep your medicine in a cool dry place where the temperature will stay below 25°C.
Do not store your medicine, or any other medicine, in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep this medicine where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or it has passed its expiry date, your pharmacist can dispose of the remaining medicine safely.
Product description
What it looks like
APO-Quetiapine 25mg tablets:
Peach, round, biconvex, film coated tablet. Engraved "APO" on one side, "QUE" over "25" on the other side.
AUST R 166082
APO-Quetiapine 100mg tablets:
Yellow, round, biconvex, film coated tablet. Engraved "APO" on one side "QUE" over "100" on the other side.
AUST R 166077
APO-Quetiapine 150mg tablets:
Pale yellow, round, biconvex, film coated tablets. Engraved "APO" on one side, "QUE" over "150" on the other side.
AUST R 166061
APO-Quetiapine 200mg tablets:
White, round, biconvex, film coated tablet. Engraved "APO" on one side, "QUE" over "200" on the other side.
AUST R 166064
APO-Quetiapine 300mg tablets:
White, capsule shaped, biconvex film coated tablet. Engraved "APO" on one side, "QUE 300" on the other side.
AUST R 166059
APO-Quetiapine is available in*:
25mg, 150mg, 200mg and 300mg: blister packs of 60
100mg: blister packs of 90
300mg: blister packs of 100
* Not all strengths, pack types and/or pack sizes may be available.
Ingredients
Each tablet contains 25mg, 100mg, 150mg, 200mg or 300mg of quetiapine (as quetiapine fumarate) as the active ingredient.
It also contains the following inactive ingredients:
- Croscarmellose sodium
- Colloidal anhydrous silica
- Fumaric acid
- Ethylcellulose
- Magnesium stearate
- Hypromellose
- Hyprolose
- Macrogol 8000
- Titanium dioxide
- Iron oxide yellow (contained in 25mg, 100mg and 150mg tablets only)
- Iron oxide red (contained in 25mg tablets only)
This medicine is gluten-free, lactose-free, sucrose-free, tartrazine-free and free of other azo dyes.
Sponsor
Apotex Pty Ltd
16 Giffnock Avenue
Macquarie Park NSW 2113
Apotex Pty Ltd is the licensee of the registered trademarks APO and APOTEX from the registered proprietor, Apotex Inc.
This leaflet was last updated in: October 2018.
Published by MIMS December 2018

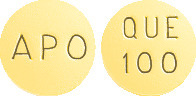

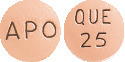




 Maintenance treatment with quetiapine was superior to placebo in increasing the time to recurrence of a depressive or a manic event (see Table 11). Patients on quetiapine also had a lower risk of experiencing a depressive or a manic event prior to week 28 and week 52 compared to patients on placebo (see Table 12).
Maintenance treatment with quetiapine was superior to placebo in increasing the time to recurrence of a depressive or a manic event (see Table 11). Patients on quetiapine also had a lower risk of experiencing a depressive or a manic event prior to week 28 and week 52 compared to patients on placebo (see Table 12).

 The magnitude of the antidepressant effect, was supported by the secondary outcome variables [Hamilton Rating Scale for Depression (HAM-D) total score, the item analyses of the MADRS and HAM-D item 1 (depressed mood) score]. Response rates (defined as (≥ 50% reduction in MADRS total score) and remission rates (defined as MADRS total score of 12 or less) were superior for quetiapine compared to placebo at week 8. The Clinical Global Impression-Severity of Illness (CGI-S) and Clinical Global Impression-Improvement (CGI-I), measures of the clinicians impression of the severity of the patients overall illness and improvement from baseline, were also assessed with quetiapine superior to placebo at week 8 in all 4 studies.
The magnitude of the antidepressant effect, was supported by the secondary outcome variables [Hamilton Rating Scale for Depression (HAM-D) total score, the item analyses of the MADRS and HAM-D item 1 (depressed mood) score]. Response rates (defined as (≥ 50% reduction in MADRS total score) and remission rates (defined as MADRS total score of 12 or less) were superior for quetiapine compared to placebo at week 8. The Clinical Global Impression-Severity of Illness (CGI-S) and Clinical Global Impression-Improvement (CGI-I), measures of the clinicians impression of the severity of the patients overall illness and improvement from baseline, were also assessed with quetiapine superior to placebo at week 8 in all 4 studies.



 Adverse events occurring at an incidence of 5% or more in any randomised treatment group from placebo controlled clinical trials in patients with bipolar I disorder treated with quetiapine as monotherapy maintenance therapy is summarised by randomised treatment in Table 4 regardless of causality.
Adverse events occurring at an incidence of 5% or more in any randomised treatment group from placebo controlled clinical trials in patients with bipolar I disorder treated with quetiapine as monotherapy maintenance therapy is summarised by randomised treatment in Table 4 regardless of causality.



 The adverse events ≥ 5% reported in a 26 week, open label clinical trial with quetiapine immediate release in children and adolescents with schizophrenia and bipolar mania were: somnolence (22.9%), headache (18.7%), sedation (14.2%), weight increased (13.4%), vomiting (10.8%), nausea (9.5%), dizziness (8.7%), fatigue (8.2%), insomnia (8.2%), increased appetite (7.1%), upper respiratory tract infection (6.8%), agitation (5.3%), irritability (5.0%), tachycardia (5.0%).
The adverse events ≥ 5% reported in a 26 week, open label clinical trial with quetiapine immediate release in children and adolescents with schizophrenia and bipolar mania were: somnolence (22.9%), headache (18.7%), sedation (14.2%), weight increased (13.4%), vomiting (10.8%), nausea (9.5%), dizziness (8.7%), fatigue (8.2%), insomnia (8.2%), increased appetite (7.1%), upper respiratory tract infection (6.8%), agitation (5.3%), irritability (5.0%), tachycardia (5.0%).
 Very rare cases of cataract have been reported in the postmarketing data, but no causal link between these reports and quetiapine has been established.
Very rare cases of cataract have been reported in the postmarketing data, but no causal link between these reports and quetiapine has been established.