What is in this leaflet
This leaflet answers some common questions about APO-Tadalafil. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
The information in this leaflet was last updated on the date shown on the final page. More recent information on this medicine may be available. Make sure you speak to your pharmacist, nurse or doctor to obtain the most up to date information on this medicine.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking APO-Tadalafil against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, talk to your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What APO-Tadalafil is used for
APO-Tadalafil is used to treat:
- erectile dysfunction, also known as impotence, in men. This is when a man cannot get, or maintain, a hard erect penis suitable for sexual activity. Following sexual stimulation, APO-Tadalafil works by helping the blood vessels in your penis to relax, allowing the flow of blood into your penis. The result of this is improved erectile function. APO-Tadalafil will only treat erectile dysfunction if you are sexually aroused. You and your partner will need to engage in foreplay, just as you would if you were not taking a medicine for erectile dysfunction.
- urinary symptoms associated with a common condition called benign prostatic hyperplasia. This is when the prostate gland gets bigger with age. Symptoms include difficulty in starting to pass urine, a feeling of not completely emptying the bladder and a more frequent need to pass urine even at night. APO-Tadalafil improves blood flow to, and relaxes the muscles of, the prostate and bladder which may reduce symptoms of benign prostatic hyperplasia.
Your doctor may have prescribed you APO-Tadalafil to treat either, or both of these conditions.
Ask your doctor if you have any questions about why APO-Tadalafil has been prescribed for you. Your doctor may have prescribed it for another reason. APO-Tadalafil is available only with a doctor's prescription.
APO-Tadalafil is not intended for use by women or by children under the age of 18 years.
The active ingredient in APO-Tadalafil tablets, tadalafil, belongs to a group of medicines called phosphodiesterase type 5 inhibitors.
Before you take APO-Tadalafil
When you must not take it
Do not take APO-Tadalafil if you are currently taking any nitrates or amyl nitrite. Nitrates are medicines used for the treatment of angina ("chest pain") or other heart conditions. APO-Tadalafil has been shown to increase the effects of these medicines.
If you are taking any form of nitrate or are unsure talk to your doctor.
Do not take APO-Tadalafil:
- if you have heart or blood vessel problems that make sexual intercourse inadvisable.
Sexual activity carries a possible risk to patients with a heart condition because it puts extra strain on the heart - if you have heart problems such as angina, arrhythmias (changes in rhythm or rate of heart beat), heart failure.
- if you have suffered a heart attack in the last 3 months
- if you have suffered a stroke in the last 6 months
- if your blood pressure is unusually high or low or is not effectively treated
- if you have vision loss in one eye because of non-arteritic interior ischaemic optic neuropathy (NAION)
- if you use recreational drugs called "poppers" or "amyl" like amyl nitrite and butyl nitrite that are normally taken through inhalation
- if you use guanylate cyclase stimulators such as riociguat, used to treat pulmonary arterial hypotension
Do not take APO-Tadalafil if you have an allergy to:
- any of the ingredients listed at the end of this leaflet
Some of the symptoms of an allergic reaction may include shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue or other parts of the body; rash, itching or hives on the skin.
Do not take this medicine after the expiry date printed on the pack. If it has expired, return it to your pharmacist for disposal.
Do not take this medicine if the packaging is torn or if the seals over the carton ends are missing or broken. There are no seals over the carton ends of the 5mg physician sample pack. If it is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking APO-Tadalafil, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
APO-Tadalafil tablets contain a small amount of lactose.
Tell your doctor if you are aged 75 years and over. You have a higher chance of experiencing side effects like dizziness and diarrhoea.
Your doctor will assess if APO-Tadalafil is suitable for you.
Tell your doctor if you have or have had any of the following medical conditions:
- liver or kidney problems
- heart problems or if you have had a heart attack
- blood vessel problems
- sickle cell anaemia (an abnormality of red blood cells)
- multiple myeloma (cancer of the bone marrow)
- leukaemia (cancer of the blood cells)
- any deformation of the penis (such as angulation, cavernosal fibrosis or Peyronie's disease)
- loss of vision in one or both eyes
If you have not told your doctor or pharmacist about any of the above, tell them before you start taking APO-Tadalafil .
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may be affected by APO-Tadalafil or may affect how it works. These include:
- Nitrates, medicine such as glyceryl trinitrate, isosorbide mononitrate and sodium nitroprusside used to treat angina and other heart conditions
- Some antibiotic medicines such as rifampicin, erythromycin and clarithromycin
- Some medicines used to treat seizures such as phenytoin, phenobarbitone and carbamazepine
- Some medicines used to treat fungal infections such as ketoconazole and itraconazole
- Protease inhibitors used to treat HIV such as ritonavir and saquinavir
- Medicines used to treat hypertension (high blood pressure) such as metoprolol, irbesartan and enalapril
- Alpha blockers (used to treat hypertension and some prostate problems) such as prazosin and doxazosin
- A 5-alpha reductase inhibitor, such as finasteride (used to treat prostate and hair loss problems)
- Warfarin, a medicine used to prevent or treat blood clots
- High doses of alcohol
- Grapefruit juice
- Other PDE5 inhibitors such as sildenafil used to treat erectile dysfunction or pulmonary arterial hypertension (high blood pressure in the blood vessels in the lungs)
- Guanylate cyclase stimulators such as riociguat, used to treat pulmonary arterial hypotension.
These medicines may be affected by APO-Tadalafil or may affect how well it works. You may need different amounts of your medicines or you may need to take different medicines.
You should not take APO-Tadalafil together with any other treatments for erectile dysfunction.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
How to take APO-Tadalafil
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
For Erectile Dysfunction
APO-Tadalafil can be taken as either on-demand dosing or once-a-day dosing.
APO-Tadalafil on-demand (10 mg and 20 mg) is intended for use prior to anticipated sexual activity and is not for continuous daily use.
APO-Tadalafil once-a-day (5 mg) is for patients who anticipate frequent use of APO-Tadalafil (i.e. at least twice weekly). Your Doctor can advise on the appropriateness of once-a-day treatment. When taken once a day, APO-Tadalafil allows you to obtain an erection, when sexually stimulated, at any time point during the 24 hours of the day. Do not take once-a-day dosing and on-demand dosing concurrently.
When taking APO-Tadalafil once-a-day (5mg), it may take up to 1 week for a full effect on Erectile Dysfunction.
For urinary symptoms associated with Benign Prostate Hyperplasia
APO-Tadalafil once-a-day (5mg) is taken daily. For urinary symptoms associated with benign prostatic hyperplasia it may take up to a week to notice an improvement and 1 month for a full effect on urinary symptoms such as; urinating too frequently during the day or at night, the need to urinate being too urgent, or too soon after relieving your bladder. APO-Tadalafil must be taken daily to maintain effect.
If you do not understand the instructions on the pack, ask your doctor or pharmacist for help.
How much to take
You should not take APO-Tadalafil more than once a day.
Always take APO-Tadalafil exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
ON-DEMAND DOSING FOR ERECTILE DYSFUNCTION
The maximum recommended dose of APO-Tadalafil is one 20 mg tablet taken before sexual activity.
If you have kidney disease, the recommended dose of APO-Tadalafil is one 10 mg tablet. This dose may be increased up to 20 mg. For patients with severe renal impairment 10 mg is the maximum recommended dose.
If you have liver disease, the recommended dose of APO-Tadalafil is one 10mg tablet. Patients with severe hepatic impairment should follow their doctor's advice.
No special dosing consideration is needed for the elderly or people with diabetes.
ONCE-A-DAY DOSING FOR ERECTILE DYSFUNCTION AND/OR URINARY SYMPTOMS ASSOCIATED WITH BENIGN PROSTATE HYPERPLASIA
The recommended dose of APO-Tadalafil is one 5 mg tablet taken once per day.
Dosage adjustments are not required in patients with kidney disease, unless you have severe renal impairment. Once-a-day dosing of APO-Tadalafil is not recommended in patients with severe renal impairment.
Patients with liver disease should follow their doctor's advice. Once-a-day dosing of APO-Tadalafil is not recommended in patients with severe hepatic impairment.
No special dosing consideration is needed for the elderly or people with diabetes.
How to take it
Swallow one tablet whole with a full glass of water.
When to take it
APO-Tadalafil can be taken with or without food or alcohol. However, drinking alcohol may affect your ability to get an erection, so avoid excessive drinking.
ON-DEMAND DOSING FOR ERECTILE DYSFUNCTION
APO-Tadalafil (10 mg or 20 mg) should be taken at least 30-60 minutes prior to anticipated sexual activity. The amount of time APO-Tadalafil takes to work varies from person to person. In some men APO-Tadalafil can work as early as 16 minutes after taking the tablet but it is recommended that you allow 1 hour the first time you take it. APO-Tadalafil has been proven to be effective for up to 36 hours. This means you can take APO-Tadalafil and it will allow you to obtain an erection when sexually stimulated, at any time during the 36 hours after taking it. Sometimes if you're feeling anxious or nervous you may not respond to the first tablet. Don't give up, it may take a few doses before you get the full benefit of APO-Tadalafil.
ONCE-A-DAY DOSING FOR ERECTILE DYSFUNCTION AND/OR URINARY SYMPTOMS ASSOCIATED WITH BENIGN PROSTATE HYPERPLASIA
APO-Tadalafil 5 mg should be taken daily at approximately the same time of day. APO-Tadalafil must be taken daily to maintain effect.
If you have any questions about taking APO-Tadalafil, ask your doctor or pharmacist.
If you take too much (overdose)
Immediately telephone your doctor or the Australian Poisons Information Centre (telephone 13 11 26) or the New Zealand National Poisons Centre (0800 POISON or 0800 764 766), or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much APO-Tadalafil. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
Symptoms of an overdose may include headache, dyspepsia (indigestion), back pain, muscular aches, nasal congestion and facial flushing.
While you are taking APO-Tadalafil
Things you must do
If you have emergency treatment for any suspected heart condition tell the emergency medical or ambulance staff that you are taking APO-Tadalafil. If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking APO-Tadalafil. Tell any other doctors, dentists and pharmacists who treat you that you are taking APO-Tadalafil.
Things you must not do
Do not take APO-Tadalafil to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Things to be careful of
Be careful driving or operating machinery until you know how APO-Tadalafil affects you.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking APO-Tadalafil.
APO-Tadalafil helps most people with erectile dysfunction or urinary symptoms associated with benign prostatic hyperplasia but it may have unwanted side effects in a few people. All medicines can have side effects.
Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by the following list of possible side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
The following side effects are usually mild and short-lived.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- facial flushing
- indigestion
- chest pain
- increased heartbeat
- back pain
- tiredness (fatigue)
- feeling sick (nausea) or vomiting
- muscle aches, including pain in the arms and legs
- stomach pain
- diarrhoea
- heart burn
- allergic reactions, including skin rash, swelling of the face and hives
- sweating
- headache or migraine
- nasal congestion
- dizziness
- fainting
- infection
- sore throat and discomfort when swallowing
- red eyes, eye pain and swelling of eyelids are uncommon
- changes in colour vision are rare
- decreases or loss of vision are very rare
- blurred vision
- bleeding nose
- prolonged erection
If you experience chest pain during or after sexual activity, stop what you are doing, sit up and sit forward.
Call an ambulance if the pain does not resolve. Do not use nitrates.
In rare instances it is possible that a prolonged and possibly painful erection may occur after taking APO-Tadalafil. Penile bleeding or presence of blood in semen may occur rarely.
If you have an erection which lasts longer than 4 hours you should contact a doctor immediately.
When taking APO-Tadalafil with large amounts of alcohol, some men may experience dizziness.
Sudden loss or decrease in hearing which may be accompanied by ringing in the ears and dizziness, loss of vision in one or both eyes and seizures have been reported in people taking APO-Tadalafil. It is not possible to determine whether these events are related directly to APO-Tadalafil, to other diseases or medications, to other factors, or to a combination of factors. If you experience these symptoms, stop taking APO-Tadalafil and contact a doctor immediately.
This is not a complete list of all possible side effects. Others may occur in some people and there may be side effects not yet known.
Potential withdrawal effects from daily use have not been examined. It is recommended that patients continue to be monitored by their doctor after discontinuation of APO-Tadalafil.
If you notice any symptoms that worry you, check with your doctor.
Ask your doctor or pharmacist if you don't understand anything on this list.
After taking APO-Tadalafil
Storage
Keep your tablets in the original pack until it is time to take them. If you take the tablets out of the pack, they may not keep as well.
Keep your tablets in a cool dry place where the temperature stays below 25°C.
Do not store APO-Tadalafil or any other medicine in the bathroom or near a sink. Do not leave it on a windowsill or in the car. Heat and dampness can destroy some medicines.
Keep your medicine where children cannot reach. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
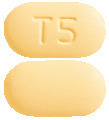
APO-Tadalafil 5 mg tablets are light yellow, capsule shaped, biconvex, bevel-edged, film-coated tablets, debossed with "T 5" on one side and plain on the other side.
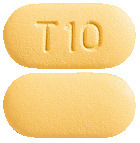
APO-Tadalafil 10 mg tablets are light yellow, capsule shaped, biconvex, bevel-edged, film-coated tablets, debossed with "T 10" on one side and plain on the other side.
APO-Tadalafil 20 mg tablets are yellow, capsule shaped, biconvex, bevel-edged, film-coated tablets, debossed with "T 20" on one side and plain on the other side.
Ingredients
Active Ingredients
- 5 mg tablet - tadalafil 5 mg
- 10 mg tablet - tadalafil 10 mg
- 20 mg tablet - tadalafil 20 mg
Other Ingredients
- croscarmellose sodium
- hypromellose
- lactose monohydrate
- magnesium stearate
- microcrystalline cellulose
- sorbitan stearate
APO-Tadalafil 20 mg tablets also contain Opadry II complete film-coating system 32K520009 Yellow.
APO-Tadalafil 10 mg tablets also contain Opadry II complete film-coating system 32K520017 Yellow.
APO-Tadalafil 5 mg tablets also contain Opadry II complete film-coating system 32K520007 Yellow.
This medicine contains lactose monohydrate.
Supplier
APO-Tadalafil is a product of:
Accord Healthcare Pty Limited
Level 24, 570 Bourke St
Melbourne
VIC 3000
Australian Registration Number
APO-Tadalafil 5 mg tablet - AUST R 282075
APO-Tadalafil 10 mg tablet - AUST R 282076
APO-Tadalafil 20 mg tablet - AUST R 282077
This leaflet was revised in August 2020
Published by MIMS September 2020





 The improvement in total IPSS in the tadalafil group compared to placebo occurred as early as 1 week in the integrated data from Studies LVHJ and LVID.
The improvement in total IPSS in the tadalafil group compared to placebo occurred as early as 1 week in the integrated data from Studies LVHJ and LVID.

 Chemical name: pyrazino[1', 2':1, 6]pyrido[3, 4-b]indole-1, 4-dione, 6-(1, 3-benzodioxol-5-yl)-2, 3, 6, 7, 12, 12a-hexahydro-2-methyl-, (6R, 12aR).
Chemical name: pyrazino[1', 2':1, 6]pyrido[3, 4-b]indole-1, 4-dione, 6-(1, 3-benzodioxol-5-yl)-2, 3, 6, 7, 12, 12a-hexahydro-2-methyl-, (6R, 12aR).