What is in this leaflet
This leaflet answers some common questions about this medicine. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking this medicine against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What this medicine is used for
The name of your medicine is APO-Temozolomide. It contains the active ingredient temozolomide.
It is used to treat:
- patients with certain types of brain tumours called glioblastoma multiforme and anaplastic astrocytoma
- adult patients with advanced metastatic malignant melanoma
Temozolomide belongs to a group of medicines called cytotoxic or chemotherapy medicines.
It works by killing cancer cells and stopping cancer cells from growing and multiplying.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed this medicine for another reason.
There is no evidence that this medicine is addictive.
This medicine is available only with a doctor's prescription.
Use in children
Temozolomide is used to treat children 3 years and older, with specific forms of brain tumour (glioblastoma multiforme or anaplastic astrocytoma, showing recurrence or progression after standard therapy).
Before you take this medicine
When you must not take it
Do not take this medicine if you or your partner are pregnant or intend to become pregnant. Temozolomide may cause birth defects if either the male or female is using this medicine at the time of conception or during pregnancy.
Therefore, female patients must have a negative pregnancy test before starting this medicine and avoid pregnancy for 6 months after discontinuation of treatment with temozolomide.
Both male and female patients and their partners should each use birth control while taking temozolomide. Male patients whose partners are already pregnant should use a condom to minimise exposure of the unborn baby to temozolomide in the sperm.
Do not take this medicine if you are breastfeeding. It is not known whether temozolomide passes into human breast milk and there is a possibility that your baby may be affected.
Do not take this medicine if you have a very low level of white blood cells, red blood cells or platelets.
Do not take this medicine if you have an allergy to:
- temozolomide
- dacarbazine (DTIC)
- any of the ingredients listed at the end of this leaflet
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you have or have had any of the following medical conditions:
- anaemia or blood clotting problems
- liver or kidney problems
- tendency to vomit frequently or experience nausea
- viruses such as cytomegalovirus and hepatitis B
Tell your doctor if you are pregnant, plan to become pregnant or are breastfeeding, or you intend to have children.
Temozolomide may cause infertility in men.
If you have not told your doctor about any of the above, tell them before you start taking this medicine.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interact with temozolomide. These include:
- other medicines used to treat cancer or that may lower your immune system
- valproic acid, used to treat epilepsy and bipolar disorder
These medicines may be affected by temozolomide or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
Other medicines not listed above may also interact with temozolomide.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
How to take this medicine
Follow carefully all directions given to you by your doctor. They may differ from the information contained in this leaflet.
If you do not understand the directions, ask your doctor or pharmacist for help.
How much to take
Your doctor will tell you how much of this medicine you should take.
This will depend on your condition and whether you are taking any other medicines.
You may be given other medications to take before or after this medicine to help stop nausea.
Newly diagnosed glioblastoma multiforme (in combination with radiotherapy):
Concomitant Phase
Your doctor will start you on a dose of temozolomide every day for 42 days (up to 49 days if needed due to radiotherapy interruption). Your treatment will then be interrupted for 4 weeks to give your body a chance to recover.
Adjuvant Phase
In the next phase, there are up to 6 treatment cycles. Each treatment cycle lasts 28 days. You will take your new dose of temozolomide once daily for the first 5 days of each cycle, followed by 23 days without temozolomide. After day 28, the next cycle will begin, in which you will again take temozolomide once daily for 5 days followed by 23 days without temozolomide. Before each new treatment cycle begins, your blood will be tested to determine if the temozolomide dose needs to be adjusted.
Recurrent gliobastoma multiforme or anaplastic astrocytoma:
Take the dose the doctor has prescribed once a day for 5 days.
Depending on your response to temozolomide, a new treatment cycle will begin each 28 days. You will then again take temozolomide once daily for 5 days.
Before each new treatment cycle, your blood will be tested to see if the dose needs to be changed.
Metastatic malignant melanoma:
Take the dose the doctor has prescribed once a day for 5 days.
Depending on your response to temozolomide, a new treatment cycle will begin each 28 days. You will then again take temozolomide once daily for 5 days.
Before each new treatment cycle, your blood will be tested to see if the dose needs to be changed.
How to take it
Swallow the capsules whole with a glass of water. Do not open or chew the capsules.
Each time you start a new treatment cycle, be sure you understand exactly how many capsules of each strength you need to take on each day of dosing.
If you are confused or unsure about how to take your dose, call your doctor for instruction before beginning the treatment cycle. Errors in how you take this medicine may have serious health consequences.
When to take it
Take your medicine at about the same time each day. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
Take this medicine without food at least one hour before a meal.
If vomiting occurs after you take this medicine, do not take another dose that day.
How long to take it for
Continue taking your medicine for as long as your doctor tells you. Your doctor will tell you when your treatment should be stopped.
If you forget to take it
If you miss a dose, take the missed dose as soon as possible during the same day. If a full day has gone by, check with your doctor.
Do not take a double dose to make up for missed doses. This may increase the chance of you experiencing side effects.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much of this medicine. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
While you are taking this medicine
Things you must do
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking this medicine.
Tell any other doctors, dentists, and pharmacists who treat you that you are taking this medicine.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking this medicine. It may affect other medicines used during surgery.
If you or your partner become pregnant or start to breastfeed while taking this medicine, tell your doctor immediately.
Tell your doctor if you become unusually pale or tired, get blood clotting problems or frequent infections while being treated with temozolomide. These could be caused by a low level of red blood cells, platelets or white blood cells in the blood. This is more common in patients over 70 years of age. Your doctor may need to change your dose of temozolomide.
If you are about to have any blood tests, tell your doctor that you are taking this medicine. It may interfere with the results of some tests.
Keep all your doctor's appointments so that your progress can be checked. Your doctor may do some tests from time to time to make sure the medicine is working and to prevent unwanted side effects.
Things you must not do
Do not open the capsules. If a capsule is damaged, avoid contact with your skin, eyes and nose. Avoid inhaling the powder. If you touch the powder or get some in your eyes or nose, wash the area with water.
Do not take this medicine to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop taking your medicine or lower the dosage without checking with your doctor.
Things to be careful of
Be careful when driving or operating machinery until you know how this medicine affects you. This medicine may cause drowsiness, sleepiness or tiredness in some people and affect alertness. If this occurs, do not drive.
Side effects
Tell your doctor as soon as possible if you do not feel well while you are taking temozolomide or if you have any questions or concerns.
All medicines can have side effects. Sometimes they are serious but most of the time they are not.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Tell your doctor if you notice any of the following:
- nausea, vomiting, feeling unwell
- tiredness, sleepiness
- constipation
- headache
- loss of appetite or weight
- diarrhoea
- fever or high temperature
- rash
- hair loss, itching
- dizziness, weakness
- general body pain
- stomach pain, indigestion
- different taste sensation
- mouth ulcers
- coughing
- sleeplessness.
Tell your doctor as soon as possible if you notice any of the following:
- frequent urination, unquenchable thirst
- shortness of breath
- tingling or numbness in hands or feet
- bruising, bleeding or being unusually pale or tired.
This could be caused by a low level of platelets or red blood cells in the blood. - new or recurring cytomegalovirus infection and return of hepatitis B
- symptoms such as fever, headache, personality change, seizures, and/or vomiting which could be associated with a brain infection caused by herpes virus
- shivering that is associated with chills and fever.
This could be sign of an infection caused by a low level of white blood cells in the blood. - development of red or purple spots under the skin.
These may be serious side effects and you may need medical attention.
The last two side effects may also take some time to occur. Therefore, even after you have finished your treatment with temozolomide, you should tell your doctor immediately if you notice these side effects.
Other side effects not listed above may occur in some patients.
If any of the following happen, tell your doctor immediately or go to Accident and Emergency at your nearest hospital:
- symptoms of an allergic reaction including cough, shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue, throat or other parts of the body; rash, itching or hives on the skin
Storage and disposal
Storage
Keep your medicine in its original packaging until it is time to take it. If you take your medicine out of its original packaging it may not keep well.
Keep your medicine in a cool dry place where the temperature will stay below 25°C.
Do not store your medicine, or any other medicine, in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep this medicine where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What APO-Temozolomide looks like
5 mg capsules:
Light green opaque, size 4, marked with black imprint "TMZ 5 mg". AUST R 231523.
20 mg capsules:
Rich yellow opaque, size 1, marked with black imprint "TMZ 20 mg". AUST R 231524.
100 mg capsules:
Flesh opaque, size 1, marked with black imprint "TMZ 100 mg". AUST R 231527.
140 mg capsules:
Powder blue opaque, size 1, marked with black imprint "TMZ 140 mg". AUST R 231528.
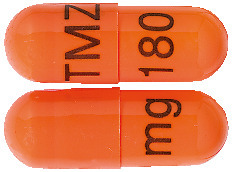
180 mg capsules:
Medium orange opaque, size 1, marked with black imprint "TMZ 180 mg". AUST R 231525.
250 mg capsules:
Buff opaque, size 0, marked with black imprint "TMZ 250 mg". AUST R 231526.
Available in bottles of 5 capsules.
Not all strengths may be available.
Ingredients
Each capsule contains 5 mg, 20 mg, 100 mg, 140 mg, 180 mg or 250 mg of temozolomide as the active ingredient.
It also contains the following inactive ingredients:
- lactose (in the 5 mg and 20 mg capsules only)
- sodium starch glycollate
- stearic acid
- tartaric acid
- microcrystalline cellulose
- colloidal anhydrous silica.
Temozolomide capsule shells contain:
- gelatin
- purified water
- titanium dioxide
- iron oxide yellow (5 mg, 20 mg and 250 mg capsules only)
- indigo carmine (5 mg and 140 mg capsules only)
- iron oxide red (100 mg and 250 mg capsules only
- iron oxide black (100 mg and 250 mg capsules only)
- sunset yellow FCF (180 mg capsules only)
- allura red AC (180 mg capsules only).
Capsule printing ink is Opacode monogramming ink S-1-277002 BLACK.
This medicine is gluten-free, sucrose-free, tartrazine-free and free of other azo dyes. Contains trace amounts of sulfites.
Sponsor
Apotex Pty Ltd
16 Giffnock Avenue
Macquarie Park NSW 2113
Australia
APO is a registered trademark of Apotex Inc.
This leaflet was last updated in:
October 2020.
Published by MIMS December 2020

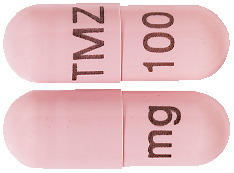
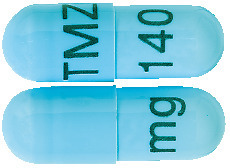







 In clinical trials, the most frequently occurring undesirable effects were gastrointestinal disturbances, specifically nausea (43%) and vomiting (36%). These effects were usually Grade 1 or 2 (mild to moderate in severity) and were either self-limiting or readily controlled with standard anti-emetic therapy. The incidence of severe nausea and vomiting was 4%. Severe myelosuppression, predominantly thrombocytopenia, was dose-limiting and occurred in 7% of all patients. Anaemia was reported in 5% of patients. Severe neutropenia and leucopenia occurred in 3% and 2% of patients, respectively.
In clinical trials, the most frequently occurring undesirable effects were gastrointestinal disturbances, specifically nausea (43%) and vomiting (36%). These effects were usually Grade 1 or 2 (mild to moderate in severity) and were either self-limiting or readily controlled with standard anti-emetic therapy. The incidence of severe nausea and vomiting was 4%. Severe myelosuppression, predominantly thrombocytopenia, was dose-limiting and occurred in 7% of all patients. Anaemia was reported in 5% of patients. Severe neutropenia and leucopenia occurred in 3% and 2% of patients, respectively.
 Chemical name: imidazo [5,1-d]-1,2,3,5- tetrazine-8-carboxamide,3,4-dihydro- 3-methyl-4-oxo.
Chemical name: imidazo [5,1-d]-1,2,3,5- tetrazine-8-carboxamide,3,4-dihydro- 3-methyl-4-oxo.