What is in this leaflet
This leaflet answers some common questions about APO-TRANEXAMIC ACID.
It does not contain all of the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have benefits and risks. Your doctor has weighed the risks of you taking APO-TRANEXAMIC ACID against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, talk to your doctor or pharmacist.
Keep this leaflet with your medicine. You may need to read it again.
What this medicine is used for
APO-TRANEXAMIC ACID is used to prevent bleeding in patients with:
Traumatic hyphaema (bleeding into the front part of the eye)
Blood clotting disorders, who are having minor surgery
Heavy periods
Hereditary angioneurotic oedema (periodic swelling of the throat)
APO-TRANEXAMIC ACID contains tranexamic acid. Tranexamic acid is an antifibrinolytic that works by slowing the processes that cause bleeding.
Before you take this medicine
When you must not take it
Do not take this medicine if:
- have an allergy to tranexamic acid or any of the ingredients listed at the end of this leaflet
- are being treated for stroke
- are being treated for blood clots in your legs, lungs or anywhere else in your body
- have a problem with colour vision that developed after you were born.
- the expiry date (Exp.) printed on the pack has passed.
- the packaging is torn or shows signs of tampering.
Do not use APO-TRANEXAMIC ACID to treat any other complaint unless your doctor tells you to.
Before you start to take it
Tell your doctor if you have any of the following:
- you, or someone in your family, has ever suffered from blood clots
- severe bruising
- kidney disease with or without blood in the urine
- irregular periods and the reason is not known
Tell your doctor if you have or have ever suffered from convulsion, fits or seizures before you start taking APO-TRANEXAMIC ACID. Convulsions, fits or seizures have been reported with APO-TRANEXAMIC ACID treatment.
Tell your doctor if you are allergic to any other medicines, foods, dyes or preservatives.
Tell your doctor if you are pregnant or plan to become pregnant. Your doctor will discuss the risks and benefits of taking APO-TRANEXAMIC ACID during pregnancy.
Tell your doctor if you are breastfeeding or wish to breastfeed. Your doctor will discuss the risks and benefits of taking APO-TRANEXAMIC ACID when breastfeeding.
If you have not told your doctor about any of the above, tell them before you start taking APO-TRANEXAMIC ACID.
Do not give this medicine to anyone else even if they have the same condition as you.
Taking other medicines
Tell your doctor if you are taking any other medicines, including any that you buy without a prescription from a pharmacy, supermarket or health food shop.
Some medicines may be affected by APO-TRANEXAMIC ACID, or may affect how well it works. These include:
- other medicines used to prevent bleeding
- medicines used to thin blood.
Your doctor can tell you what to do if you are taking any of these medicines.
If you are not sure whether you are taking any of these medicines, check with your doctor or pharmacist. Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking APO-TRANEXAMIC ACID.
How to take this medicine
Follow carefully all directions given to you by your doctor or pharmacist. Their instructions may be different to the information in this leaflet.
How much to take
Traumatic hyphaema (bleeding to the front part of the eye)
Take two or three tablets every 8 hours, for six to seven days. Swallow the tablets with water.
Heavy periods
Take two or three tablets four times a day for four days. Start taking the tablets when you first notice the bleeding. Take APO-TRANEXAMIC ACID tablets for the first four days of your period. See your doctor for a check up after three months of treatment. If the bleeding is not reduced, talk to your doctor. Swallow the tablets with water.
Hereditary Angioneurotic Oedema (periodic swelling of the throat)
The usual dose is two or three tablets, 2 to 3 times a day. Your doctor will tell you how long to take the tablets. Swallow the tablets with water.
For those with a clotting disorder, having minor surgery
The usual dose is two or three tablets, 2 to 3 times a day. Your doctor will tell you how long to take the tablets. Swallow the tablets with water.
The directions your doctor gives you should be strictly followed.
If you do not understand the instructions in this leaflet, ask your doctor or pharmacist for help.
If you forget to take it
Take your APO-TRANEXAMIC ACID Tablets as soon as you remember and then go back to taking it as normally would.
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Do not try to make up for missed doses by taking more than one dose at a time because this may increase the chance of you getting an unwanted side effect.
If you have any questions about this, check with your doctor or pharmacist.
If you take too much (overdose)
Symptoms from taking too much APO-TRANEXAMIC ACID include:
- dizziness
- headache
- nausea
- diarrhoea
- low blood pressure
- convulsions, fits or seizures.
Immediately telephone your doctor, or the Poisons Information Centre (telephone 13 11 26), or go to Accident and Emergency at the nearest hospital, if you think you or anyone else may have taken too much APO-TRANEXAMIC ACID. Do this even if there are no signs of discomfort or poisoning.
You may need urgent medical attention.
While you are taking this medicine
Things you must do
Before starting any new medicine, tell your doctor or pharmacist that you are taking APO-TRANEXAMIC ACID.
Tell all the doctors, dentists and pharmacists who are treating you that you are taking APO-TRANEXAMIC ACID.
Possible side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking APO-TRANEXAMIC ACID.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if you notice any of the following and they worry you:
- nausea
- vomiting
- diarrhoea.
There are the more common side effects of APO-TRANEXAMIC ACID. Mostly these are mild and short-lived.
Following cardiac surgery, total knee replacement or total hip replacement surgery, tell your doctor or nurse immediately if you experience any of the following:
- irregular and often rapid heart beat
- heart attack
- slow or irregular heart beat
- cardiogenic shock caused by very low blood pressure. The symptoms are dizziness and light headedness, rapid, weak pulse, white skin, sweating, restlessness, loss of consciousness, fainting, rapid, shallow breathing, cold clammy skin and weakness
- stroke. The symptoms of stroke are numbness or weakness of the arms or legs, headache, dizziness and confusion, visual disturbance, difficulty swallowing, slurred speech and loss of speech
- kidney problems where you pass little or no urine, drowsiness, nausea, vomiting and breathlessness
- difficulty breathing
- a condition called deep vein thrombosis (DVT). The symptoms of DVT are pain and swelling in the large veins, usually in your legs. DVT may lead to complications such as blood clots in your lungs
- bowel infarction caused by a restriction of blood supply to the bowels. You may experience severe abdominal pains and may pass bloody stools.
These are serious side effects. You may need urgent medical attention.
Tell your doctor immediately or go to Accident and Emergency at the nearest hospital if you notice any of the following:
- unexpected pain
- unexpected swelling in your legs or arms
- giddiness or dizziness
- allergic skin reactions
- changes in your eyesight
- convulsions, fits or seizures.
These may be serious side effects. You may need urgent medical attention.
Other side effects not listed above may also occur in some patients. Tell your doctor if you notice anything that is making you feel unwell.
Storage and disposal
Storage
Keep APO-TRANEXAMIC ACID where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Keep your tablets in the pack until it is time to take them. If you take the tablets out of the pack they may not keep well.
Keep your tablets in a cool dry place where the temperature stays below 25°C.
Do not store APO-TRANEXAMIC ACID or any other medicine in the bathroom or near a sink.
Do not leave APO-TRANEXAMIC ACID in the car or on window sills. Heat and dampness can destroy some medicines.
Disposal
If your doctor tells you to stop taking APO-TRANEXAMIC ACID, or your tablets have passed their expiry date, ask your pharmacist what to do with any that are left over.
Product description
What APO-TRANEXAMIC ACID tablet looks like
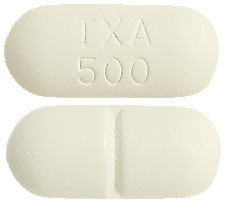
500mg tablet: Tranexamic acid is a white to slightly yellowish, film coated, capsule shaped, biconvex, 18 mm x 8 mm, with a break-line and embossed "TXA 500"
Each pack contains 100 tablets.
Ingredients
The active ingredient in APO-TRANEXAMIC ACID is tranexamic acid. Each 500 mg tablet contains 500 mg of tranexamic acid.
The tablet also contains:
- microcrystalline cellulose
- povidone
- croscarmellose sodium
- colloidal anhydrous silica
- purified talc
- magnesium stearate
- titanium dioxide
- macrogol 8000
- vanillin
- eudragit E100.
The tablets do not contain gluten, lactose, sucrose, tartrazine or any other azo dyes.
Australian Registration Numbers
APO-Tranexamic acid 500mg tablets (blister pack): AUST R 272732
APO-Tranexamic acid 500mg tablets (bottle pack): AUST R 272727
Sponsor
Southern Cross Pharma Pty Ltd
Suite 2 Level 2
19-23 Prospect Street
VIC 3128
APO and APOTEX are registered trademarks of Apotex Inc.
Distributor
Arrotex Pharmaceuticals
15 – 17 Chapel St
Cremorne VIC 3121
www.arrotex.com.au
This leaflet was last updated in:
February 2023
Published by MIMS June 2023