What is in this leaflet
This leaflet answers some common questions about ARIDON APN. It does not contain all the available information.
It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking ARIDON APN against the benefits they expect it will have for you.
If you have any concerns about using this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What ARIDON APN is used for
ARIDON APN are used to treat mild, moderate and severe Alzheimer’s disease, also called dementia of the Alzheimer’s type.
ARIDON APN will not cure this disease, but should help your memory and improve your thinking capacity.
This medicine belongs to a group of medicines called acetylcholinesterase inhibitors. They are thought to work by increasing the level of a chemical called acetylcholine in the brain.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
This medicine is not addictive.
This medicine is only available with a doctor's prescription.
Before you take ARIDON APN
When you must not take it
Do not take ARIDON APN if you have an allergy to:
- any medicine containing donepezil hydrochloride
- piperidine derivatives
- any of the ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue or other parts of the body; rash, itching or hives on the skin.
Do not give ARIDON APN to children. The safety and effectiveness of ARIDON APN in children have not been established.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should be taking ARIDON APN, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you plan to go to hospital for surgery that requires a general anaesthetic.
Tell your doctor if you have or have had any of the following medical conditions:
- heart problems
- stomach problems, particularly gastric or duodenal ulcer
- seizures or fits (epilepsy)
- asthma or obstructive pulmonary disease
- loss of memory or other mental capacity due to stroke or blood vessel problems
- a tendency towards aggressive behaviour.
Tell your doctor if you are pregnant or plan to become pregnant or are breast-feeding. Your doctor can discuss with you the risks and benefits involved.
If you have not told your doctor about any of the above, tell him/her before you start taking ARIDON APN.
Taking other medicines
Tell your doctor if you are taking any other medicines, including any that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and ARIDON APN may interfere with each other. These include:
- any other medicine for dementia
- some medicines used to relieve stomach cramps or spasms, Parkinson’s disease or travel sickness
- some medicines used to treat difficulty in passing urine
- non-steroidal anti-inflammatory drugs (NSAIDs) - medicines used to treat arthritis, pain or inflammation
- some medicines used to relax muscles
- some medicines used to treat high blood pressure or fast heart beat
- some medicines used to treat irregular heart beat such as quinidine
- some medicines for treating asthma, diarrhoea, depression, schizophrenia and related mental conditions, or used in general anaesthesia
- carbamazepine, phenobarbitone or phenytoin, medicines used to treat epilepsy
- ketoconazole, a medicine used to treat fungal infections
- rifampicin, an antibiotic used to treat tuberculosis
- dexamethasone, a corticosteroid medicine.
These medicines may be affected by ARIDON APN or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
If you are not sure if you are taking any of these medicines, ask your doctor or pharmacist. Your doctor or pharmacist has more information on medicines to be careful with or avoid while taking ARIDON APN.
If you have not told your doctor or pharmacist about these things, tell them before you start taking ARIDON APN.
How to Take ARIDON APN
Follow all directions given to you by your doctor and pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the packaging, ask your doctor or pharmacist for help.
How much to take
The usual starting dose for ARIDON APN is one 5 mg tablet each day.
After 1-month, your doctor will assess your response and may increase your dose to one 10 mg tablet a day.
However, depending on your condition and how you react to the medicine, your doctor may ask you to take some other dose.
How to take it
Swallow the tablets whole with a full glass of water.
When to take it
Take the tablet every night just before you go to bed. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
ARIDON APN can be taken with or without food.
How long to take it
Continue taking your medicine for as long as your doctor tells you. This medicine helps to control your condition, but does not cure it. It is important to keep taking your medicine even if you feel well.
It may take several weeks for ARIDON APN to take effect, so do not be discouraged if you do not see an improvement straight away.
If you forget to take it
If you forget to take a tablet, just take one tablet the following day at the usual time then continue as normal.
Do not take a double dose to make up for the dose you missed. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you forget to take it for more than 1-week, call your doctor before taking any more.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre - the telephone number in Australia is 131 126 and in New Zealand is 0800 POISON or 0800 764 766 or go to Accident and Emergency (Casualty) at your nearest hospital, if you think that you or anyone else may have taken too much ARIDON APN. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
Symptoms of an overdose may include feeling sick in the stomach, vomiting, increased sweating or saliva production. You may also have a slow heart beat, feel dizzy, have trouble breathing, faint, have fits, feel weak or not be able to control your bowel motions or passing of urine.
While you are taking it
Things you must do
Tell all doctors, dentists and pharmacists who are treating you that you are taking ARIDON APN.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking ARIDON APN.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking this medicine. It may affect other medicines used during surgery. Your doctor may ask you to stop taking ARIDON APN a few days before you have surgery.
Tell your doctor immediately if you become pregnant while taking ARIDON APN. If you are a woman of child-bearing age, you should avoid becoming pregnant while taking ARIDON APN.
Keep all of your doctor’s appointments so that your progress can be checked. Your doctor may do some tests from time to time to make sure the medicine is working and to prevent unwanted side effects.
Things you must not do
Do not give this medicine to anyone else, even if they have the same condition as you.
Do not use ARIDON APN to treat any other complaints unless your doctor says to.
Things to be careful of
Be careful driving or operating machinery until you know how ARIDON APN affects you. This medicine may cause fatigue, dizziness and muscle cramps especially at the start of treatment or when the dose is increased. If you have any of these symptoms, do not drive, operate machinery or do anything else that could be dangerous.
In addition, Alzheimer’s disease may affect your ability to drive or operate machinery. Ask your doctor whether it is safe for you to continue to drive or operate machinery.
Side effects
Tell your doctor as soon as possible if you do not feel well while you are taking ARIDON APN.
This medicine helps most people with Alzheimer’s disease, but it may have unwanted side effects in a few people. All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- heartburn, indigestion, or stomach pain
- headache, dizziness
- difficulty in sleeping
- unusual tiredness
- feeling sick, diarrhoea, vomiting,
- loss of appetite, weight loss
- bruising
- muscle cramps, joint pain
- tingling or numbness of the hands or feet
- depression, unusual dreams
- agitation, aggressive behaviour
- difficulty in urinating or passing urine more often.
The above list includes the more common side effects of your medicine. They are usually mild and short-lived.
Tell your doctor as soon as possible if you notice any of the following:
- seeing, feeling or hearing things that are not there
- trembling and shaking of the hands and fingers, shuffling walk and stiffness of the arms and legs
- severe upper stomach pain, often with nausea, vomiting and fever.
The above list includes serious side effects which may require medical attention.
If any of the following happen, tell your doctor immediately or go to Accident and Emergency at your nearest hospital:
- any breathing problems
- sudden signs of allergy such as rash, itching or hives, swelling of the face, lips, tongue or other parts of the body, shortness of breath, wheezing or difficulty breathing.
- fainting, especially if you have a slow or irregular heart beat
- vomiting blood or material that looks like coffee grounds
- black sticky bowel motions (stools)
- convulsions or fits
- weakness, shortness of breath, yellowing of the skin, dark brown urine and stomach pain
- sudden increase in body temperature, extremely high blood pressure and severe convulsions.
The above list includes very serious side effects. You may need urgent medical attention or hospitalisation.
Tell your doctor if you notice anything else that is making you feel unwell.
Other side effects not listed above may also occur in some people.
After Taking ARIDON APN
Storage
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Keep ARIDON APN in a cool, dry place where the temperature stays below 25°C.
Do not store it or any other medicine in the bathroom or near a sink. Do not leave it in the car or on window sills. Heat and dampness can destroy some medicines.
Keep your tablets in their blister pack until it is time to take them. If you take the tablets out of the pack they may not keep well.
Disposal
If your doctor tells you to stop taking ARIDON APN, or the tablets have passed their expiry date, ask your pharmacist what to do with any left over.
Product Description
What it looks like
- ARIDON APN 5 – White to off-white, round, bevelled edge, biconvex, film coated tablets with inscription "5" on one side and plain on other side.
- ARIDON APN 10 -Yellow, round, biconvex, film coated tablets with inscription "10" on one side and plain on other side.
Ingredients
Active ingredient(s):
- ARIDON APN 5 – 5 mg donepezil hydrochloride per tablet
- ARIDON APN 10 – 10 mg donepezil hydrochloride per tablet
Inactive ingredients:
- lactose monohydrate
- maize starch
- microcrystalline cellulose
- hyprolose
- magnesium stearate
- OPADRY Complete Film Coating System OY-38951 WHITE (ARTG No. 106399) [5 mg tablets only]
- OPADRY Complete Film Coating System 03B32654 YELLOW (ARTG No. 106398) [10 mg tablets only]
ARIDON APN contains sugars as lactose. ARIDON APN do not contain gluten.
ARIDON APN are presented in blister packs containing 28 tablets.
Sponsor
Arrow Pharma Pty Ltd
15-17 Chapel Street
Cremorne VIC 3121
ARIDON APN 5:
AUST R No. 167193
ARIDON APN 10:
AUST R No. 167190
Date of revision:
October 2021
Published by MIMS November 2021

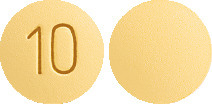



 Figure 2 illustrates the cumulative percentages of patients from each of the three treatment groups who had attained the measure of improvement in ADAS-cog score shown on the X axis. Three change scores, (7-point and 4-point reductions from baseline or no change in score) have been identified for illustrative purposes and the percent of patients in each group achieving that result is shown in this inset table.
Figure 2 illustrates the cumulative percentages of patients from each of the three treatment groups who had attained the measure of improvement in ADAS-cog score shown on the X axis. Three change scores, (7-point and 4-point reductions from baseline or no change in score) have been identified for illustrative purposes and the percent of patients in each group achieving that result is shown in this inset table.

 Following 3 weeks of placebo washout, scores on the ADAS-cog for both the donepezil hydrochloride treatment groups increased, indicating that discontinuation of donepezil hydrochloride resulted in a loss of its treatment effect. The duration of this placebo washout period was not sufficient to characterise the rate of loss of the treatment effect, but the 30-week study (see above) demonstrated that treatment effects associated with the use of donepezil hydrochloride abate within 6 weeks of treatment discontinuation.
Following 3 weeks of placebo washout, scores on the ADAS-cog for both the donepezil hydrochloride treatment groups increased, indicating that discontinuation of donepezil hydrochloride resulted in a loss of its treatment effect. The duration of this placebo washout period was not sufficient to characterise the rate of loss of the treatment effect, but the 30-week study (see above) demonstrated that treatment effects associated with the use of donepezil hydrochloride abate within 6 weeks of treatment discontinuation.
 In both studies, patient age, sex and race were not found to predict the clinical outcome of donepezil hydrochloride treatment.
In both studies, patient age, sex and race were not found to predict the clinical outcome of donepezil hydrochloride treatment.

 Individual study description and efficacy results for the 26-week and the two 24-week studies are shown below.
Individual study description and efficacy results for the 26-week and the two 24-week studies are shown below.
 Long term efficacy data are provided by an open label extension of this study. After 36 weeks of donepezil treatment, the mean SIB value remained at or near the baseline level, suggesting no further decline in cognitive functions.
Long term efficacy data are provided by an open label extension of this study. After 36 weeks of donepezil treatment, the mean SIB value remained at or near the baseline level, suggesting no further decline in cognitive functions.
 Molecular formula: C24H29NO3HCl.
Molecular formula: C24H29NO3HCl.