1 Name of Medicine
Atovaquone and proguanil hydrochloride.
2 Qualitative and Quantitative Composition
AtovaquoPro Lupin 250/100 and AtovaquoPro Lupin 62.5/25 are fixed combination products containing atovaquone and proguanil hydrochloride.
Each AtovaquoPro Lupin 250/100 tablet contains 250 mg atovaquone and 100 mg proguanil hydrochloride. Each AtovaquoPro Lupin 62.5/25 tablet contains 62.5 mg atovaquone and 25 mg proguanil hydrochloride.
For the full list of excipients, see Section 6.1 List of Excipients.
3 Pharmaceutical Form
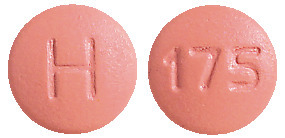
AtovaquoPro Lupin 250/100.
Pink, round, biconvex, film-coated tablets, debossed with 'H' on one side and '175' on the other side.
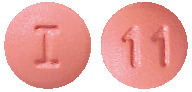
AtovaquoPro Lupin 62.5/25.
Pink, round, biconvex, film-coated tablets, debossed with 'I' on one side and '11' on the other side.4.1 Therapeutic Indications
AtovaquoPro Lupin is indicated for:
Prophylaxis of Plasmodium falciparum malaria in adults and children ≥ 11 kg.
Treatment of Plasmodium falciparum malaria in adults and children aged 3 years or older.
4.2 Dose and Method of Administration
The daily dose should be taken with food or a milky drink at the same time each day.
In the event of vomiting within 1 hour of dosing, a repeat dose should be taken.
AtovaquoPro Lupin 250/100 or AtovaquoPro Lupin 62.5/25 tablets should preferably be swallowed whole. If difficulties are encountered when dosing young children, the tablet(s) may be crushed and added to a small amount of milk, all of which should be consumed immediately.
Prophylaxis.
Prophylaxis should start 1 to 2 days before entering a malaria-endemic area and be continued daily until seven days after leaving the area.
If patients are unable to tolerate food, AtovaquoPro Lupin tablets should be administered, but systemic exposure of atovaquone will be reduced.
Dosage in adults.
One AtovaquoPro Lupin 250/100 tablet daily.
Dosage in children.
See Table 1.

Treatment.
Dosage in adults.
Four tablets as a single dose for three consecutive days.
Dosage in children.
See Table 2.

Dosage in the elderly (prophylaxis and treatment).
A pharmacokinetic study indicates that no dosage adjustments are needed in the elderly (see Section 5.2 Pharmacokinetic Properties).
Dosage in hepatic impairment (prophylaxis and treatment).
A pharmacokinetic study indicates that no dosage adjustments are needed in patients with mild to moderate hepatic impairment. No studies have been conducted in patients with severe hepatic impairment (see Section 5.2 Pharmacokinetic Properties).
Dosage in renal impairment (prophylaxis and treatment).
Pharmacokinetic studies indicate that no dosage adjustments are needed in patients with mild to moderate renal impairment. In patients with severe renal impairment (creatinine clearance < 30 mL/min) alternatives to AtovaquoPro Lupin should be recommended for the treatment of acute P. falciparum malaria whenever possible (see Section 4.4 Special Warnings and Precautions for Use; Section 5.2 Pharmacokinetic Properties). For prophylaxis of P. falciparum malaria in patients with severe renal impairment see Section 4.3 Contraindications.4.3 Contraindications
AtovaquoPro Lupin is contraindicated in individuals with known hypersensitivity to atovaquone or proguanil hydrochloride or to any component of the formulation.
AtovaquoPro Lupin is contraindicated for prophylaxis of P. falciparum malaria in patients with severe renal impairment (creatinine clearance < 30 mL/min).
4.4 Special Warnings and Precautions for Use
Atovaquone/proguanil has not been evaluated for the treatment of cerebral malaria or other severe manifestations of complicated malaria including hyperparasitaemia, pulmonary oedema or renal failure.
Safety and efficacy of atovaquone/proguanil for the treatment and prophylaxis of malaria in paediatric patients who weigh less than 11 kg have not been established.
In the event of recrudescent infections due to P. falciparum or failure of chemoprophylaxis, patients should be treated with a different blood schizonticide.
Parasite relapse occurred commonly when P. vivax malaria was treated with atovaquone/proguanil alone. Travellers with intense exposure to P. vivax or P. ovale, and those who develop malaria caused by either of these parasites, will require additional treatment with a drug such as primaquine, that is active against hypnozoites.
Persons taking atovaquone/proguanil for prophylaxis or treatment of malaria should take a repeat dose if they vomit within 1 hour of dosing. In the event of diarrhoea, normal dosing should be continued. Absorption of atovaquone may be reduced in patients with diarrhoea or vomiting, but diarrhoea or vomiting was not associated with reduced efficacy in clinical trials of atovaquone/proguanil for malaria prophylaxis. However, as with other antimalarial agents, patients with diarrhoea or vomiting should be advised to continue to comply with personal protection measures (e.g. repellents, bednets).
In patients with acute malaria who present with diarrhoea or vomiting, alternative therapy should be considered. If AtovaquoPro Lupin is used to treat malaria in these patients, parasitaemia should be closely monitored.
The co-administration of atovaquone/proguanil with other antimalarial drugs has not been evaluated.
Parasitaemia should be closely monitored in patients receiving concurrent metoclopramide or tetracycline (see Section 4.5 Interactions with Other Medicines and Other Forms of Interactions).
The concomitant administration of atovaquone/proguanil tablets and rifampicin or rifabutin is not recommended (see Section 4.5 Interactions with Other Medicines and Other Forms of Interactions).
Use in hepatic impairment.
Pharmacokinetic studies indicate that no dosage adjustments are needed in patients with mild to moderate hepatic impairment. Atovaquone/proguanil has not been specifically studied in patients with severe hepatic impairment.
Use in renal impairment.
Pharmacokinetic studies indicate that no dosage adjustments are needed in patients with mild to moderate renal impairment. In patients with severe renal impairment (creatinine clearance < 30 mL/min) alternatives to AtovaquoPro Lupin for treatment of acute P. falciparum malaria should be recommended whenever possible (see Section 4.2 Dose and Method of Administration; Section 4.3 Contraindications; Section 5.2 Pharmacokinetic Properties).
Use in the elderly.
Pharmacokinetic studies indicate that no dosage adjustments are needed in the elderly.
Paediatric use.
Dosage recommendations in children are based on body weight (see Section 4.2 Dose and Method of Administration).
Effects on laboratory tests.
No data available.4.5 Interactions with Other Medicines and Other Forms of Interactions
Proguanil may potentiate the anticoagulant effect of warfarin and other coumarin based anticoagulants. The mechanism of this potential drug interaction has not been established. Caution is advised when initiating or withdrawing malaria prophylaxis or treatment with AtovaquoPro Lupin in patients on continuous treatment with coumarin based anticoagulants.
Concomitant treatment with tetracycline, metoclopramide, rifampicin and rifabutin have been associated with significant decreases in plasma concentration of atovaquone (see Section 4.4 Special Warnings and Precautions for Use). Concomitant administration of tetracycline and atovaquone/proguanil reduced the plasma concentrations of atovaquone but had no effect on the efficacy of atovaquone/proguanil in curing Plasmodium falciparum malaria.
Concomitant administration of atovaquone and indinavir results in a 23% decrease in the Cmin of indinavir in healthy individuals. Caution should be exercised when prescribing atovaquone with indinavir due to the decrease in trough levels of indinavir.
In clinical studies of atovaquone in the treatment of diseases other than malaria, small decreases in plasma concentrations were associated with concomitant use of paracetamol, benzodiazepines, aciclovir, opiates, cephalosporins, anti-diarrhoeal agents and laxatives. The implications of these observations for use with AtovaquoPro Lupin are not known. In the same series of studies, the following medications were not associated with a change in steady state plasma concentrations of atovaquone: fluconazole, clotrimazole, ketoconazole, antacids, systemic corticosteroids, non-steroidal anti-inflammatory drugs, anti-emetic drugs (excluding metoclopramide) and H2-antagonists.
There is no information available on whether interactions occur between atovaquone and terfenadine or cisapride.
Atovaquone is highly protein bound (> 99%) but does not displace other highly protein bound drugs in vitro, indicating significant drug interactions arising from drug displacement are unlikely.
Co-administration of efavirenz with atovaquone/proguanil may result in a decrease in exposure to atovaquone and proguanil. When given with efavirenz or boosted protease-inhibitors, atovaquone concentrations have been observed to decrease as much as 75%. Since decreased concentrations of atovaquone and proguanil may result in a decrease of antimalarial efficacy, concomitant administration should be avoided whenever possible.
4.6 Fertility, Pregnancy and Lactation
Effects on fertility.
There are no data on the effect of atovaquone on human fertility. Data from animal studies show that atovaquone does not affect reproductive potential or performance at oral doses of up to 1000 mg/kg (approximately 6.5 times human exposure at the maximum recommended clinical treatment dose, based on AUC). A study in rats showed no impairment of male or female fertility at oral proguanil doses up to 16 mg/kg/day (approximately 0.03 times human exposure at the recommended clinical treatment dose, based on AUC). However, there is some evidence from published animal studies that proguanil and/or its main metabolite, cycloguanil, may cause impairment of fertility/early embryonic loss. No fertility studies have been performed in animals with atovaquone in combination with proguanil.
Findings in repeat dose studies with the atovaquone and proguanil hydrochloride combination were entirely proguanil related. As proguanil has been used extensively and safely in the treatment and prophylaxis of malaria at doses similar to those used in AtovaquoPro Lupin, these findings are considered of little relevance in the clinical situation.
(Category B2)
The safety of the drug combination in human pregnancy has not been established. There is no information on effects of atovaquone administration during human pregnancy. Foetal death and malformation have rarely been reported in association with the use of proguanil.
The relationship of these events to proguanil is not certain, and the overall number of reported events is low, given that the drug has been used in pregnant women for many years. Foetal loss is a known complication of Plasmodium falciparum malaria in pregnancy.
The proguanil component of AtovaquoPro Lupin acts by inhibiting parasitic dihydrofolate reductase. There are no clinical data indicating that folate supplementation diminishes drug efficacy. For women of childbearing age receiving folate supplements to prevent neural tube birth defects, such supplements may be continued while taking AtovaquoPro Lupin.
Embryofoetal development studies in animals with the combination of atovaquone and proguanil did not indicate any teratogenic potential in rats at doses up to 50:20 mg/kg/day (approximately 5 times the human exposure to atovaquone and 0.3 times human exposure to proguanil, based on treatment AUCs), nor in rabbits at doses up to 100:40 mg/kg/day (approximately 1 times the human exposure to atovaquone and 0.5 times the exposure to proguanil, based on treatment AUCs). In rabbits given atovaquone alone at 1200 mg/kg/day (approximately 1.4 times the estimated human exposure during treatment of malaria), an increased incidence of resorptions and decreased length and weight of foetuses was noted. These effects were observed only in the presence of maternal toxicity.
In a peri-postnatal study in rats dosed with proguanil alone up to 16 mg/kg/day (0.03 times the human exposure, based on treatment AUC), no treatment-related effects were seen in reproductive or other parameters in the F0, F1 and F2 generations.
However, as animal studies are not always predictive of human response, the use of AtovaquoPro Lupin in pregnancy should only be considered if the expected benefit to the mother outweighs the risk to the foetus.
It is not known whether atovaquone is excreted into human milk. In a rat study, the atovaquone concentrations in milk were 30% of the concurrent atovaquone concentrations in maternal plasma. Proguanil is excreted in human milk in small quantities. Breastfeeding is not recommended during treatment with AtovaquoPro Lupin.4.7 Effects on Ability to Drive and Use Machines
There have been no studies to investigate the effect of atovaquone and proguanil on driving performance or the ability to operate machinery. Detrimental effect on such activities is not predicted from the pharmacology of the component drugs.
4.8 Adverse Effects (Undesirable Effects)
As AtovaquoPro Lupin contains atovaquone and proguanil hydrochloride, the type and severity of adverse reactions associated with each of the compounds may be expected. However, at the doses employed for both treatment and prophylaxis of malaria, adverse reactions are generally mild and of limited duration. There is no evidence of added toxicity following concurrent administration of the two compounds.
Prophylaxis.
Individuals > 40 kg.
The nature and frequency of adverse reactions reported in clinical trials of atovaquone/proguanil tablets for the prophylaxis of malaria in individuals weighing > 40 kg were similar to those reported with placebo or the active comparator drug (mefloquine or chloroquine/proguanil). However, patients receiving atovaquone/proguanil tablets had fewer neuropsychiatric and gastrointestinal adverse reactions than patients receiving mefloquine and chloroquine/proguanil respectively. Overall, atovaquone/proguanil has a better safety profile than mefloquine or chloroquine/proguanil (see Tables 3 and 4).


Individuals 11-40 kg.
The incidence of adverse reactions reported in clinical trials using atovaquone/proguanil tablets (62.5 mg/25 mg) for the prophylaxis of malaria in individuals weighing 11-40 kg were similar to those reported with placebo (MALB3003 and MAL30015). In studies MAL30010 and MAL30012, the incidence of drug-related adverse events was higher in the chloroquine/proguanil group (15% vs 11% for atovaquone/proguanil) during active treatment. Due to the low number of patients in study MAL30010 (n = 12), no drug-related adverse events were reported by the mefloquine recipients. Tables 5 and 6 list the common drug-related adverse reactions reported during chemoprophylaxis by treatment group.


Treatment.
The nature and frequency of adverse experiences reported in controlled clinical trials of atovaquone and proguanil hydrochloride for the treatment of malaria were generally similar in patients treated with the combination or with a comparator antimalarial drug. This suggests that the adverse experiences are largely due to the disease rather than to study drugs (see Table 7).
 A similar profile of clinical adverse events was reported in children with malaria treated with atovaquone and proguanil hydrochloride in phase III trials as occurred in the adult studies.
A similar profile of clinical adverse events was reported in children with malaria treated with atovaquone and proguanil hydrochloride in phase III trials as occurred in the adult studies.
Regardless of attributability, the following were also commonly reported (> 2%) in children: dehydration, tinnitus and anorexia.
Of the seven severe or treatment limiting adverse experiences reported in clinical trials with atovaquone/proguanil, three were considered to be treatment related; two were reports of nausea and/or vomiting and one report of an anaphylactic reaction. During clinical trials, two subjects receiving atovaquone monotherapy experienced psychiatric symptoms. One subject had a history of psychiatric illness and the other a history of drug and alcohol abuse. Two subjects receiving atovaquone/proguanil hydrochloride had seizures; in one of these cases the patient successfully continued treatment. Both subjects had a prior history of seizures and the investigators did not consider the events attributable to the atovaquone/proguanil treatment.
Adverse events are listed below by system organ class and frequency. Frequencies are defined as: very common (≥ 1/10), common (≥ 1/100 and < 1/10), uncommon (≥ 1/1,000 and < 1/100), rare (≥ 1/10,000 and < 1/1,000) and very rare (< 1/10,000). Very common, common and uncommon events were determined from clinical trial data. Rare and very rare events were generally derived from spontaneous data. The frequency classification "not known" has been applied to those events where a frequency could not be estimated from the available data.
A summary of adverse events identified during world-wide post-approval use of atovaquone/proguanil or its components, atovaquone and proguanil hydrochloride is provided below.
Blood and lymphatic system disorders.
Common: anaemia1, neutropenia2.
Not known: pancytopenia in patients with severe renal impairment4.
Immune system disorders.
Not known: angioedema4, anaphylaxis3, vasculitis.
Metabolism and nutritional disorders.
Common: anorexia1, hyponatraemia2.
Uncommon: elevated amylase levels2 occurred in patients treated with atovaquone.
Psychiatric disorders.
Rare: hallucinations1.
Nervous system disorders.
Very common: headache1.
Common: insomnia1, dizziness1.
Gastrointestinal disorders.
Very common: abdominal pain1, nausea2, vomiting1, diarrhoea1.
Uncommon: stomatitis1.
Not known: gastric intolerance4, oral ulceration4.
Hepatobiliary disorders.
Common: elevated liver enzyme levels2.
Not known: hepatitis3, cholestasis.
Clinical trial data for atovaquone/proguanil tablets indicated that abnormalities in liver function tests were reversible and not associated with untoward clinical events.
Skin and subcutaneous tissue disorders.
Common: rash1.
Uncommon: hair loss1, urticaria1.
Not known: Stevens-Johnson syndrome3, erythema multiforme3.
General disorders and administration site conditions.
Common: fever1.
Respiratory, thoracic and mediastinal disorders.
Common: cough1.
1 Frequency calculated from atovaquone/proguanil clinical trials.
2 Frequency taken from atovaquone label. Patients participating in clinical trials with atovaquone have received higher doses and have often had complications of advance human immunodeficiency virus (HIV) disease. Therefore, the causal relationship between the adverse experiences and atovaquone is difficult to evaluate. These events may have been seen at a lower frequency or not at all in clinical trials with atovaquone/proguanil.
3 Observed from post-marketing spontaneous reports and the frequency is therefore not known.
4 Observed with proguanil and the frequency is therefore not known.
Reporting suspected adverse effects.
Reporting suspected adverse reactions after registration of the medicinal product is important. It allows continued monitoring of the benefit-risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions at www.tga.gov.au/reporting-problems.4.9 Overdose
In cases of suspected overdose, symptomatic and supportive therapy should be given as appropriate.
For information on the management of overdose, contact the Poisons Information Centre on 13 11 26 (Australia).
5 Pharmacological Properties
5.1 Pharmacodynamic Properties
Mechanism of action.
The constituents of AtovaquoPro Lupin, atovaquone and proguanil hydrochloride, interfere with two different pathways in the biosynthesis of pyrimidines, required for nucleic acid replication. The mechanism of action of atovaquone against P. falciparum is via inhibition of mitochondrial electron transport, at the level of the cytochrome bc1 complex, and collapse of mitochondrial membrane potential. One mechanism of action of proguanil, via its metabolite cycloguanil, is inhibition of dihydrofolate reductase, which disrupts deoxythymidylate synthesis. Proguanil also has antimalarial activity independent of its metabolism to cycloguanil, and proguanil, but not cycloguanil, is able to potentiate the ability of atovaquone to collapse mitochondrial membrane potential in malaria parasites. This latter mechanism may explain the synergy seen when atovaquone and proguanil are used in combination, as in AtovaquoPro Lupin.
Microbiology.
Atovaquone is active against Plasmodium spp (in vitro IC50 against P. falciparum 0.23-1.43 nanogram/mL).
The antimalarial activity of proguanil is exerted via the primary metabolite cycloguanil (in vitro IC50 against various P. falciparum strains of 4-20 nanogram/mL). Some activity of proguanil and another metabolite, 4-chlorophenylbiguanide, is seen in vitro at 0.6-3.0 microgram/mL.
In in vitro studies of P. falciparum, the combination of atovaquone and proguanil was shown to be synergistic. This enhanced efficacy was also demonstrated in clinical studies.
Clinical trials.
The safety and effectiveness of atovaquone/proguanil tablets (250 mg/100 mg) and atovaquone/proguanil tablets (62.5 mg/25 mg) have been established in studies of up to 12 weeks in adult and paediatric subjects.
Prophylaxis of malaria (individuals > 40 kg).
The safety and efficacy of atovaquone/proguanil tablets (250 mg/100 mg) in the prophylaxis of P. falciparum malaria was demonstrated in five randomised, double-blind clinical studies. Three placebo-controlled parallel group studies were conducted in residents of malaria-endemic areas (MALB2001, MALB3001 and MALB3003), and two active-controlled studies were conducted in non-immune travellers (MALB30010 and MALB30011).
There were 473 patients in placebo-controlled studies, 232 of whom received one atovaquone/proguanil tablet (250 mg/100 mg) daily for 10-12 weeks of chemoprophylaxis, and 241 received placebo. Prevention of parasitaemia was the primary endpoint in the studies. Atovaquone/proguanil had an overall efficacy of 97% (range 95-100%) for prevention of P. falciparum parasitaemia and an adverse event profile similar to placebo.
MALB3003 included 204 children (weighing 11-40 kg) who received a lower dose of atovaquone/proguanil or placebo based on body weight (see Prophylaxis of malaria (individuals 11-40 kg)). There were 1,975 patients in active controlled studies, 993 of whom received one atovaquone/proguanil tablet (250 mg/100 mg) daily at the recommended dose (see Section 4.2 Dose and Method of Administration), 471 received mefloquine weekly (1 to 3 weeks before until 4 weeks after travel) and 511 patients received chloroquine weekly (1 week before until 4 weeks after travel) plus daily proguanil (1-2 days before until 4 weeks after travel). Frequency of adverse events was the primary endpoint and development of confirmed falciparum malaria within 60 days after leaving the malaria-endemic area was the secondary endpoint in the studies. No patients receiving atovaquone/proguanil or mefloquine contracted malaria (efficacy 100%), and 3 patients receiving chloroquine/proguanil contracted malaria (efficacy at least 70%). Patients receiving atovaquone/proguanil experienced fewer neuropsychiatric and gastrointestinal adverse reactions than patients receiving mefloquine and chloroquine/proguanil respectively.
Prophylaxis of malaria (individuals 11-40 kg).
The efficacy and safety of atovaquone/proguanil tablets (62.5 mg/25 mg) in the prophylaxis of P. falciparum malaria in patients weighing 11-40 kg was demonstrated in two randomised, placebo-controlled, double-blind studies of 12 week duration conducted in residents of malaria endemic areas. A total of 534 patients (11-40 kg) were enrolled in the studies, of which 264 received the recommended dose of atovaquone/proguanil tablets (62.5 mg/25 mg) based on body weight: 11-20 kg - 1 x 62.5 mg/25 mg tablet (containing 62.5 mg atovaquone and 25 mg proguanil hydrochloride); 21-30 kg - 2 x 62.5 mg/25 mg tablets; 31-40 kg - 3 x 62.5 mg/25 mg tablets (MALB3003 and MAL30015).
In the combined data from the two studies (per protocol population), only one of 238 patients (0.4%) in the atovaquone/proguanil group developed P. falciparum parasitaemia during chemoprophylaxis over 12 weeks, compared with 50 of 245 (20.4%) patients in the placebo group. The protective efficacy of atovaquone/proguanil was calculated to be 97.9% in this population. The safety findings with regard to adverse events during chemosuppression showed no differences between atovaquone/proguanil and placebo.
The safety profile of atovaquone/proguanil was assessed in two active-controlled studies in travellers to malaria endemic areas (Studies MAL30010 - mefloquine and MAL30012 - chloroquine/proguanil) (see Section 4.8 Adverse Effects (Undesirable Effects)). With respect to efficacy, in combined data from the two active-controlled studies (n = 186; 93 in the atovaquone/proguanil group), there was no confirmed cases of P. falciparum during chemoprophylaxis or in follow-up to Day 60.
Treatment of malaria.
Eight clinical studies (5 controlled and 3 uncontrolled) were conducted in 1,115 patients of atovaquone and/or proguanil hydrochloride administered for the treatment of falciparum malaria. Studies in children were conducted at doses of atovaquone and proguanil hydrochloride based on body weight; 466 patients (adults and children) received concurrent atovaquone and proguanil hydrochloride at the recommended dose (see Section 4.2 Dose and Method of Administration).
The primary efficacy endpoint was the proportion of evaluable patients cured of acute malaria. Cure was defined by clearance of asexual parasitaemia within 7 days of initiation of treatment, without subsequent recrudescence during the 28 day follow-up period.
In the controlled clinical trials, the study population included only patients with uncomplicated falciparum malaria. The comparator was standard antimalarial therapy within the country in which the study was conducted. Treatment with combination of atovaquone and proguanil hydrochloride was curative in 98% of evaluable patients (combined result). The concurrent administration of atovaquone and proguanil hydrochloride was more efficacious in three studies and of equivalent efficacy in two trials as the respective comparator antimalarial regimen (Table 8).
 In uncontrolled studies conducted in Thailand using the recommended dose of atovaquone and proguanil hydrochloride, the cure rate of malaria was 100% in adults (n = 24, P. falciparum) and 100% in children (n = 26, P. falciparum).
In uncontrolled studies conducted in Thailand using the recommended dose of atovaquone and proguanil hydrochloride, the cure rate of malaria was 100% in adults (n = 24, P. falciparum) and 100% in children (n = 26, P. falciparum).
5.2 Pharmacokinetic Properties
There are no pharmacokinetic interactions between atovaquone and proguanil at the recommended dose. In clinical trials, trough levels of atovaquone, proguanil and cycloguanil in children (weighing 11-40 kg) are within the effective range observed in adults after adjusting for bodyweight.
Absorption.
Atovaquone is a highly lipophilic compound with low aqueous solubility and poor oral bioavailability that varies with dose and diet.
Although there are no atovaquone bioavailability data in healthy subjects, in HIV-infected patients the absolute bioavailability of a 750 mg single dose of atovaquone tablets taken with food is 21% (90% CI:17%-27%).
Dietary fat taken with atovaquone increases the rate and extent of absorption. When taken with a standard breakfast containing 23 g of fat, AUC was increased 2-3 times and Cmax 5 times compared with fasting. Patients are recommended to take AtovaquoPro Lupin with food or a milky drink (see Section 4.2 Dose and Method of Administration).
Proguanil hydrochloride is rapidly and extensively absorbed regardless of food intake. Peak plasma concentrations occur between 2-4 hours after a single 200 mg dose. The absolute bioavailability is not known.
In a comparative bioavailability study in healthy adult volunteers, atovaquone/proguanil tablets administered as a single dose was bioequivalent to separate tablets of atovaquone 250 mg and proguanil hydrochloride 100 mg given concomitantly. In healthy adult subjects treated for 3 days, the pharmacokinetics of atovaquone, and proguanil and its metabolite cycloguanil, were not modified when atovaquone and proguanil were given alone or in combination as atovaquone/proguanil.
Distribution.
Atovaquone is highly protein bound (> 99%) but does not displace other highly protein bound drugs in vitro, indicating significant drug interactions arising from displacement are unlikely.
Following oral administration, the volume of distribution of atovaquone in adults and children is approximately 7 to 8 L/kg.
Proguanil is 75% protein bound. Following oral administration, the volume of distribution of proguanil in adults is 25 L/kg. In children (weighing 11-40 kg), the volume of distribution is approximately 27 to 30 L/kg.
In human plasma, the protein binding of atovaquone or proguanil was unaffected by the presence of the other drug.
Metabolism.
There is no evidence that atovaquone is metabolised. Greater than 90% of atovaquone is eliminated unchanged in the faeces with negligible excretion in urine.
Proguanil hydrochloride is partially metabolised to cycloguanil and 4-chlorophenyl biguanide with less than 40% being excreted unchanged in urine. These metabolites are also excreted in the urine. Conversion of proguanil to cycloguanil is mediated in the liver by cytochrome P450 3A4 and 2C19. Conversion of proguanil to cycloguanil may be reduced in some individuals, due to genetic polymorphism of the metabolising enzyme. During administration with atovaquone/proguanil, at the recommended doses, proguanil metabolism status appears to have no implications for treatment or prophylaxis of malaria.
Excretion.
The elimination half-life of atovaquone is about 2-3 days in adults and 1-2 days in children.
Following oral administration, the clearance of atovaquone in adults and children (weighing 40 kg) is approximately 0.04 to 0.05 L/h/kg. In children (weighing 11-40 kg), the clearance is approximately 0.12 to 0.05 L/h/kg, respectively.
Following oral administration, the clearance of proguanil in adults is 1.3 L/h/kg. In children (11-40 kg body weight) after adjusting for differences in body weight, clearance is higher in an 11 kg child (0.12 L/h/kg) and decreases with increasing weight to 0.05 L/h/kg in a 40 kg child.
In both adults and children, the elimination half-life for proguanil or cycloguanil is about 12-15 hours.
Pharmacokinetics in the elderly.
There is no clinically significant change in the average rate or extent of absorption of atovaquone or proguanil between elderly and young patients. Systemic availability of cyloguanil is higher in the elderly compared with young patients, but there is no clinically significant change in its elimination half-life (see Section 4.2 Dose and Method of Administration).
Pharmacokinetics in renal impairment.
In patients with mild to moderate renal impairment, oral clearance and/or AUC data for atovaquone, proguanil and cycloguanil are within the range of values observed in patients with normal renal function. Atovaquone Cmax and AUC are reduced in patients with severe renal impairment. The elimination half-lives for proguanil and cycloguanil are prolonged in patients with severe renal impairment with corresponding increases in AUC, resulting in the potential of drug accumulation with repeated dosing (see Section 4.2 Dose and Method of Administration, Section 4.3 Contraindications; Section 4.4 Special Warnings and Precautions for Use).
Pharmacokinetics in hepatic impairment.
In patients with mild to moderate hepatic impairment, there is no clinically significant change in exposure to atovaquone compared with healthy patients. In patients with mild to moderate hepatic impairment there is an increase in proguanil AUC with no change in its elimination half-life and there is a decrease in Cmax and AUC for cycloguanil. No data are available in patients with severe hepatic impairment (see Section 4.2 Dose and Method of Administration; Section 4.4 Special Warnings and Precautions for Use).
5.3 Preclinical Safety Data
Genotoxicity.
There was no evidence that either atovaquone or proguanil alone were mutagenic in bacterial and mammalian cell gene mutation assays in vitro, and in mouse bone marrow micronucleus assays for chromosome damage in vivo. Cycloguanil, an active metabolite of proguanil, was negative in a bacterial mutagenicity assay but positive in both a mammalian cell mutagenicity assay and a mouse micronucleus test. As the genotoxicity of cycloguanil is prevented or moderated by the co-administration of folinic acid, it appears to be related to the inhibition of mammalian dihydrofolate reductase, causing a reduction in the nucleotide pool and a consequent perturbation of DNA synthesis rather than a direct interaction with DNA. Neither proguanil nor cycloguanil is likely to present a genotoxic risk at clinical exposure levels. Mutagenicity studies have not been performed with atovaquone in combination with proguanil.
Carcinogenicity.
Oncogenicity studies of atovaquone alone in mice showed an increased incidence of hepatocellular adenomas and carcinomas at all dose levels tested, yielding exposures approximately 5 to 8 times the average steady-state plasma concentrations in humans during prophylaxis of malaria. The pattern of associated histological findings observed in the liver, is consistent with a species specific, non-genotoxic, neoplastic response. Studies in rats at oral dose levels of up to 500 mg/kg/day were negative. Atovaquone is unlikely to present a carcinogenic risk to humans at therapeutic doses.
Oncogenicity studies on proguanil alone showed no evidence of carcinogenicity in rats and mice at doses resulting in exposures approximately equal to those obtained in humans during prophylaxis of malaria but considerably below exposures obtained during treatment of malaria. Carcinogenicity studies have not been conducted with atovaquone in combination with proguanil.6 Pharmaceutical Particulars
6.1 List of Excipients
Both tablets also contain: hyprolose, microcrystalline cellulose, povidone, sodium starch glycollate, macrogol 400, magnesium stearate, macrogol 8000, poloxamer, colloidal anhydrous silica and iron oxide red.
6.2 Incompatibilities
See Section 4.5 Interactions with Other Medicines and Other Forms of Interactions.
6.3 Shelf Life
In Australia, information on the shelf life can be found on the public summary of the Australian Register of Therapeutic Goods (ARTG). The expiry date can be found on the packaging.
6.4 Special Precautions for Storage
Store below 25°C.
6.5 Nature and Contents of Container
Each strength is provided in PVC/aluminium foil blister packs or -aluminium/aluminium foil blister packs in the following pack sizes:
AtovaquoPro Lupin 250/100.
12 and 24 tablets.
AtovaquoPro Lupin 62.5/25.
12, 24 and 60 tablets.
AtovaquoPro Lupin 250/100 and AtovaquoPro Lupin 62.5/25 are also supplied in high density polyethylene (HDPE) bottles containing 30 and 100 tablets.
Not all strengths, presentations and/or pack sizes may be distributed in Australia.
6.6 Special Precautions for Disposal
In Australia, any unused medicine or waste material should be disposed of by taking to your local pharmacy.
6.7 Physicochemical Properties
Atovaquone is virtually insoluble in water (less than 2 x 10-4 mg/mL) and slightly soluble (1.7 mg/mL) in 0.1 M sodium hydroxide.
Proguanil hydrochloride is slightly soluble at 1 part in 110 parts of water and is sparingly soluble in alcohol (1 part in 40 parts of alcohol).
Chemical structure.
Atovaquone.
 Chemical name: trans-2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthoquinone.
Chemical name: trans-2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthoquinone.
Molecular formula: C22H19ClO3.
Molecular weight: 366.84.
Proguanil hydrochloride.
 Chemical name: 1-(4-chlorophenyl)-5-isopropyl-biguanide hydrochloride.
Chemical name: 1-(4-chlorophenyl)-5-isopropyl-biguanide hydrochloride.
Molecular formula: C11H16ClN5.
Molecular weight: 290.20.
CAS number.
Atovaquone.
95233-18-4.
Proguanil hydrochloride.
637-32-1.7 Medicine Schedule (Poisons Standard)
Schedule 4 - Prescription Only Medicine.







 A similar profile of clinical adverse events was reported in children with malaria treated with atovaquone and proguanil hydrochloride in phase III trials as occurred in the adult studies.
A similar profile of clinical adverse events was reported in children with malaria treated with atovaquone and proguanil hydrochloride in phase III trials as occurred in the adult studies. In uncontrolled studies conducted in Thailand using the recommended dose of atovaquone and proguanil hydrochloride, the cure rate of malaria was 100% in adults (n = 24, P. falciparum) and 100% in children (n = 26, P. falciparum).
In uncontrolled studies conducted in Thailand using the recommended dose of atovaquone and proguanil hydrochloride, the cure rate of malaria was 100% in adults (n = 24, P. falciparum) and 100% in children (n = 26, P. falciparum). Chemical name: trans-2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthoquinone.
Chemical name: trans-2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthoquinone. Chemical name: 1-(4-chlorophenyl)-5-isopropyl-biguanide hydrochloride.
Chemical name: 1-(4-chlorophenyl)-5-isopropyl-biguanide hydrochloride.