What is in this leaflet
Read this leaflet carefully before taking AVAPRO. This leaflet answers some common questions about AVAPRO. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist. Do not throw this leaflet away. You may need to refer to it again later.
Always follow the instructions that your doctor and pharmacist give you about AVAPRO. If you have any concerns about taking AVAPRO, ask your doctor or pharmacist.
What is AVAPRO
AVAPRO is a trade name (manufacturer's name) for the medicine, irbesartan. This medicine is in the tablets your doctor has prescribed for you.
What AVAPRO is used for
AVAPRO lowers high blood pressure, which doctors call hypertension
Your doctor measured your blood pressure and found it to be too high. Everyone has blood pressure. This pressure helps get your blood all around your body. Your blood pressure may be different at different times of the day, depending on how busy or worried you are. You have hypertension (high blood pressure) which means your blood pressure stays high, even when you are calm and relaxed.
There are usually no symptoms of high blood pressure. The only way of knowing that you have hypertension is to have your blood pressure checked on a regular basis. High blood pressure, if not treated, can damage blood vessels in several organs such as the heart, the kidneys, the brain and the eyes. This may lead to heart attacks, heart or kidney failure, strokes, or blindness. There are usually no symptoms of high blood pressure before damage occurs, so your doctor needs to measure your blood pressure to see if it is too high.
High blood pressure can be treated and controlled with medicines such as AVAPRO. Your doctor may also have recommended that you adjust your lifestyle to help to lower your high blood pressure (losing weight, avoiding smoking, reducing alcohol consumption and restricting the amount of salt in the diet). Your doctor may also have encouraged the practice of regular, mild (not strenuous) exercise such as walking, swimming, etc.
AVAPRO is also used in the treatment of kidney disease in patients with high blood pressure and type 2 diabetes.
What AVAPRO does and how it works
AVAPRO belongs to a group of medicines known as angiotensin-II receptor antagonists. Angiotensin II is a substance produced in the body which causes blood vessels to tighten. AVAPRO blocks angiotensin-II and therefore relaxes your blood vessels. This helps to lower your blood pressure.
AVAPRO slows the decrease of kidney function in patients with high blood pressure and type 2 diabetes.
Your doctor may have prescribed AVAPRO for another use. If you want more information, ask your doctor.
When you must not take AVAPRO
Do not take AVAPRO if:
- you are pregnant (or think you may be pregnant) or are planning to become pregnant
Your baby may absorb this medicine in the womb and there is a possibility of harm to the baby. - you are breast-feeding
It is not known if AVAPRO passes into breast milk, therefore it is not recommended to be taken while you are breast-feeding. - you are allergic to irbesartan or to any of the ingredients listed under Product Description at the end of this leaflet.
- you are diabetic or have kidney problems and are being treated with an ACE inhibitor, any aliskiren-containing medicines or a group of medicines known as AIIRAs (medicines also used to treat high blood pressure);
- the packaging is torn or shows signs of tampering
- the expiry date on the pack has passed
If you take this medicine after the expiry date has passed, it may not work.
If you are not sure if you should start taking AVAPRO, talk to your doctor.
AVAPRO should not be given to children.
Before you start to take AVAPRO
Tell your doctor if:
- you are or intend to become pregnant or plan to breast-feed
AVAPRO should not be used during pregnancy or while breast-feeding - you have recently had excessive vomiting or diarrhoea
- you suffer from any medical conditions especially:
- kidney problems, or you have had a kidney transplant or dialysis
- heart problems
- liver problems, or have had liver problems in the past
- diabetes
- high levels of potassium in your blood - you are strictly restricting your salt intake
- you are lactose intolerant or have had any allergies to any other medicine or any other substances, such as foods, preservatives or dyes.
Be sure you tell your doctor about any of these things before you take any AVAPRO.
Taking AVAPRO with other medicines
Tell your doctor if you are taking or intend to take any other medicines, including medicines that you buy without a prescription from your pharmacy, supermarket or health food shop.
It is especially important that you tell your doctor if you are taking, or plan to take any of the following:
- other tablets for high blood pressure
- fluid tablets or diuretics
- lithium or lithium containing medicines (for example Lithicarb)
- potassium tablets (for example Span-K, Slow-K, Mag-K)
- potassium containing salt substitutes (for example Pressor-K)
- anti-inflammatory medicines (these are used to relieve pain, swelling and other symptoms of inflammation, including arthritis) and include nonsteroidal anti-inflammatory agents - NSAIDs (for example Voltaren, Indocid, ibuprofen) and COX-2 inhibitors (for example Celebrex).
Taking a combination of Avapro and an anti-inflammatory medicine, alone or with a thiazide diuretic (fluid tablet) may damage your kidneys. It may also reduce the effect AVAPRO has on reducing blood pressure. - a medicine containing aliskiren. Taking Avapro with aliskiren may affect your blood pressure, electrolyte balance and your kidney function
Your doctor will decide whether your treatment needs to be altered or whether you should have check ups or blood tests more frequently
How to take AVAPRO
The tablets should be taken regularly as directed by your doctor. The tablets should be swallowed with a glass of water.
How much to take:
Your doctor will tell you how many tablets to take each day.
Usually patients start with one 150 mg tablet once a day, however some patients may need a lower starting dose. Your doctor will tell you if this is necessary. The full blood pressure lowering effect of AVAPRO should be reached about 4-6 weeks after starting treatment.
Depending on how your blood pressure responds, your daily dose of AVAPRO may need to be increased. Most patients take either 150mg or 300mg each day.
In patients with high blood pressure and type 2 diabetes, 300mg once daily is the preferred maintenance dose for the treatment of associated kidney disease.
Follow all directions given to you by your doctor carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the box, ask your doctor or pharmacist for help.
When to take it:
You should try to take your daily dose of AVAPRO at about the same time each day. Taking your AVAPRO tablets at the same time each day will have the best effect.
It does not matter whether you take AVAPRO tablets before or after food.
How long to take it:
AVAPRO helps to control your high blood pressure, but it does not cure it. Therefore AVAPRO must be taken every day. Continue taking AVAPRO until your doctor tells you to stop.
If you miss a dose:
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to. Otherwise, take it as soon as you remember, and go back to taking your tablets as you would normally.
If you are not sure whether to skip the dose, talk to your doctor or pharmacist.
Do not take a double dose to make up for the missed dose.
If you have trouble remembering to take your tablets, ask your pharmacist for some hints.
If you take too much AVAPRO (overdose)
Immediately telephone your doctor or Poisons Information Centre (telephone 131126) or go to the Accident and Emergency Department at your nearest hospital, if you or anyone else may have taken too much AVAPRO. Do this even if there are no signs of discomfort or poisoning.
If you take too many AVAPRO tablets you will probably feel lightheaded or dizzy.
While you are using AVAPRO
Things you must do:
- If you become pregnant while taking AVAPRO tell your doctor immediately.
- Have your blood pressure checked when your doctor tells you to, to make sure AVAPRO is working.
- If you are about to start on any new medicine, tell your doctor and pharmacist that you are taking AVAPRO.
- Get up slowly when getting out of bed or standing up.
You may feel light-headed or dizzy while taking AVAPRO, especially if you are also taking a diuretic (fluid tablet). This may become worse if you stand up quickly as your blood pressure may fall. Standing up slowly, especially when you get up from bed or chairs, will help your body get used to the change in position and blood pressure. This problem is not common. If it occurs talk to your doctor. - If you plan to have surgery or other treatment (even at the dentist) that needs a general anaesthetic, tell your doctor or dentist that you are taking AVAPRO.
- Make sure you drink enough water during exercise and hot weather when you are taking AVAPRO, especially if you sweat a lot.
If you do not drink enough water while taking AVAPRO, you may faint or feel light-headed or sick. This is because your body does not have enough fluid and your blood pressure is low. If you continue to feel unwell, tell your doctor. - If you have excessive vomiting and/or diarrhoea while taking AVAPRO, tell your doctor.
This can also mean that you are losing too much water and your blood pressure may become too low.
Things you must not do:
- Do not give AVAPRO tablets to anyone else, even if they have the same condition as you.
Things to be careful of:
- Be careful driving or operating machinery until you know how AVAPRO affects you.
As with many other medicines used to treat high blood pressure, AVAPRO may cause dizziness or light-headedness in some people. Make sure you know how you react to AVAPRO before you drive a car, operate machinery or do anything else that could be dangerous if you are dizzy or light-headed.
If you drink alcohol, dizziness or light-headedness may be worse.
Side effects
AVAPRO helps most people with high blood pressure, but it may have unwanted side effects in a few people. All medicines can have side effects. Sometimes they are serious, most of the time they are not.
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking AVAPRO.
Tell your doctor if you notice any of the following and they worry you -
- headache
- dizziness or light-headedness (vertigo)
- unusual tiredness or weakness, fatigue
- nausea/vomiting
- These are common side effects. They are generally mild and do not normally require treatment to be interrupted.
Tell your doctor immediately if you notice any of the following-
- skin rash or itchiness
- aching muscles or aching joints, not caused by exercise
- muscle pain
- buzzing, ringing or other persistent noise in the ears
- yellowing of the skin and/or eyes, also called jaundice
- symptoms that may indicate kidney disease, such as passing little or no urine, drowsiness, nausea, vomiting, breathlessness, loss of appetite and weakness
- symptoms that may indicate high potassium levels in the blood such as nausea, diarrhoea, muscle weakness and change in heart rhythm
- symptoms that may indicate low platelet count such as easy or excessive bruising, bleeding from gums or nose, prolonged bleeding from cuts and blood in urine or stools.
These are serious side effects. Skin rash and itchiness may be symptoms of an allergic reaction. You may need medical attention.
These side effects are not common.
If any of the following happen, stop taking AVAPRO and tell your doctor immediately or go to Accident and Emergency Department at your nearest hospital -
- swelling to the face, lips, tongue or throat which may cause difficulty in swallowing or breathing.
- severe and sudden onset of pinkish, itchy swellings on the skin, also called hives.
These are serious side effects. If you have them you may have had a serious allergic reaction to AVAPRO. You may need urgent medical attention or hospitalisation.
These side effects are very rare.
Other side effects not listed above may occur in some patients. If you notice any other unwanted effects, you should tell your doctor or pharmacist and ask for their advice.
After using AVAPRO
Storage:
Keep your AVAPRO tablets in the blister pack until it is time to take them. If you take the tablets out of the blister pack they may not keep well.
Keep AVAPRO tablets in a cool dry place where the temperature stays below 25°C. Do not store AVAPRO or any other medicine in the bathroom or near a sink. Do not leave them near a radiator, in a car on hot days or on a window sill.
Heat and dampness can destroy some medicines.
Keep all medicines out of the reach of children.
A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal:
If your doctor tells you to stop taking AVAPRO or the tablets have passed their expiry date, ask your pharmacist what to do with any tablets that are left over.
Product description
What it looks like
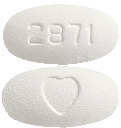
AVAPRO 75 mg tablets - white to off-white, oval, film-coated tablet with a heart shape on one side and "2871" on the other. Pack size: 30 tablets.
AUST R 101730
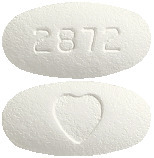
AVAPRO 150 mg tablets - white to off-white, oval, film-coated tablet with a heart shape on one side and "2872" on the other. Pack size: 30 tablets.
AUST R 101734
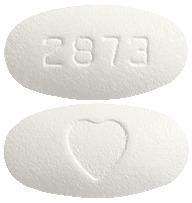
AVAPRO 300 mg tablets - white to off-white, oval, film-coated tablet with a heart shape on one side and "2873" on the other. Pack size: 30 tablets.
AUST R 101736
Active ingredients
AVAPRO 75 mg tablets - 75 mg irbesartan per tablet.
AVAPRO 150 mg tablets - 150 mg irbesartan per tablet.
AVAPRO 300 mg tablets - 300 mg irbesartan per tablet.
Other Ingredients
AVAPRO tablets also contain carnauba wax, croscarmellose sodium, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, silicon dioxide, and OPADRY II complete film coating system 32F38977 WHITE.
Sponsored by
sanofi-aventis australia pty ltd
12-24 Talavera Road
Macquarie Park, NSW 2113.
Date of Preparation: April 2020.
This information in no way replaces the advice of your doctor or pharmacist.
AVAPRO® is a registered sanofi-aventis Trademark.
avapro-ccdsv14-cmiv18-29apr20
Published by MIMS June 2020

 Adverse reactions that occurred in 2 or more hypertensive patients in clinical trials involving 3396 patients have been classified using standard terminology and in the following listing are categorised by body system and listed in order of decreasing frequency according to the following definitions: common adverse reactions are those occurring on one or more occasions in at least 1/100 but less than 1/10 patients; uncommon adverse reactions are those occurring in at least 1/1000 but less than 1/100 patients; rare adverse reactions are those occurring in less than 1/1000 patients.
Adverse reactions that occurred in 2 or more hypertensive patients in clinical trials involving 3396 patients have been classified using standard terminology and in the following listing are categorised by body system and listed in order of decreasing frequency according to the following definitions: common adverse reactions are those occurring on one or more occasions in at least 1/100 but less than 1/10 patients; uncommon adverse reactions are those occurring in at least 1/1000 but less than 1/100 patients; rare adverse reactions are those occurring in less than 1/1000 patients. Irbesartan: C25H28N6O.
Irbesartan: C25H28N6O.