WHAT IS IN THIS LEAFLET
This leaflet answers some common questions about Biltricide. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking Biltricide against the benefits they expect it will have for you.
As Biltricide is a prescription medicine, it should only be used under medical supervision.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
WHAT BILTRICIDE IS USED FOR
Biltricide is used for the treatment of schistosomiasis or bilharziasis, which is a chronic parasitic infestation in humans caused by blood flukes (worms). The flukes live in freshwater snails and you may have been infected if you swam or waded in water infested by larvae released from the snails. They are found in Africa (including Madagascar and Mauritius), the Middle East, India, Southeast Asia and South America.
The larvae penetrate the skin, most commonly on the feet, and mature into adult worms in the urinary bladder or the gut.
A rash may occur soon after infestation. Common symptoms of acute infestation are fever, night sweats, lethargy, headache, abdominal pain, loss of appetite, weight loss and a non-productive cough. You may pass blood in the urine.
Infestation with schistosoma parasites may cause no symptoms. However, they may also lead to chronic liver disease or chronic disorders of the urinary tract.
Eggs of the adult worms can occasionally be found in the brain or the spinal cord causing paralysis, or in the eyes, affecting your eyesight.
Biltricide kills blood flukes by causing an immediate contraction and paralysis of the parasite; it also stops the parasite from being able to absorb and use sugars. The parasite then disintegrates with the help of the white blood cells and is passed from the body.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
BEFORE YOU TAKE BILTRICIDE
When you must not take it
Do not take Biltricide if you have an allergy to:
- praziquantel, the active ingredient in Biltricide
- any of the ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin.
Do not take Biltricide if you are taking rifampicin (an antibiotic used to treat tuberculosis or leprosy), dexamethasone (a corticosteroid) or anti-epileptic medicines.
Do not take Biltricide if the infestation is found in your eyes, because killing the parasite may cause blindness.
Do not take this medicine after the expiry date printed on the pack and bottle. The expiry date is printed on the carton and on each vial after “EXP” (e.g. 11 18 refers to November 2018). The expiry date refers to the last day of that month. If it has expired return it to your pharmacist for disposal.
Do not take this medicine if the packaging is torn or shows signs of tampering. If the packaging is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you have or have had any of the following medical conditions:
- any heart problems
- any kidney disease that causes you to pass little or no urine
- any problems with your liver, particularly, if your liver has been infected by blood flukes
- history of epilepsy
- skin nodules (slightly elevated lesions on or in the skin).
Use of Biltricide may be associated with deterioration of your medical condition with symptoms similar to an allergic reaction. This is mainly seen in the acute phase of schistosomiasis (where the worms begin to produce eggs). This can lead to potentially life-threatening events such as lung failure, encephalopathy (disease of the brain), and/or cerebral vasculitis (narrowing or blockage of blood vessels in the brain).
If you take Biltricide for an infestation in your brain or spinal cord, you may get severe headaches and fits. If the doctor wants to treat you for your infestation in the brain, you should be taken to hospital so that a specialist can monitor your treatment.
Tell your doctor if you are pregnant or plan to become pregnant. Your doctor can discuss with you the risks and benefits of using Biltricide during pregnancy.
Tell your doctor if you are breastfeeding or intend to breastfeed. The active ingredient in Biltricide passes into breast milk and there is a possibility that your baby may be affected. You should not breastfeed your baby on the day you take Biltricide or for 3 days afterwards.
Your doctor may want to monitor you carefully or hospitalise you while you are taking Biltricide.
If you have not told your doctor or pharmacist about any of the above, tell them before you start taking Biltricide.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any you have bought at your pharmacy, supermarket or health food shop.
Do not take Biltricide if you are taking:
- rifampicin (an antibiotic used to treat tuberculosis and leprosy).
Some medicines and Biltricide may interfere with each other. These include:
- dexamethasone (an anti-inflammatory and immunosuppressant)
- medicines for treating epilepsy
- cimetidine (a medicine used to treat high acid levels in your stomach, reflux and ulcers)
- chloroquine (an antimalarial medicine)
- ketoconazole or itraconazole (medicines used to treat fungal infections)
- erythromycin (an antibiotic used to treat bacterial infections)
- grapefruit juice.
These medicines may be affected by Biltricide or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
HOW TO TAKE BILTRICIDE
How much to take
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions printed on the pharmacist label, ask your doctor or pharmacist for help.
You must take all the Biltricide tablets prescribed by your doctor or pharmacist to kill the type of blood fluke you have. Your doctor will prescribe a dose specifically for you. It is important to take all the tablets your doctor prescribed.
The dose prescribed will usually be 20 mg for every kg of your body weight taken three times in the one day every four hours.

How to take it
Swallow the tablets with a full glass of water.
The tablet can be divided into 4 parts.
Each part of the tablet has 150 mg of the active ingredient, allowing a precise dose to be given, depending on your weight.
If you need to take ¼ of a tablet, it is easier if you break the tablet at one of the outer grooves. The simplest way to break the tablet is to put your thumbnail in the groove of the tablet.
When to take it
Take your medicine at about the same time each day. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
Swallow the tablets preferably after eating a meal.
If you forget to take it
Take the dose as soon as you remember.
If it is almost time for the next dose, wait until it is time for your next dose, take your next dose when you are meant to and seek your doctor’s advice.
Do not take a double dose to make up for the dose that you missed. If you have trouble remembering when to take your medicine, ask your doctor or pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (Australia: 13 11 26 or New Zealand: 0800 POISON or 0800 764 766) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much Biltricide. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
If you take too many Biltricide tablets, you should take a laxative, which works quickly.
WHILE YOU ARE TAKING BILTRICIDE
Things you must do
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking Biltricide.
Tell all the doctors, dentists and pharmacists who are treating you that you are taking Biltricide.
If you are on corticosteroid therapy or if you are about to start taking any new medicine, especially medicines used to treat tuberculosis, leprosy, epilepsy, malaria, stomach acidity, reflux or ulcers, tell your doctor or pharmacist that you are taking Biltricide. See “BEFORE YOU TAKE BILTRICIDE” for examples of these medicines.
Things you must not do
Do not take Biltricide to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop taking your medicine or lower the dosage without checking with your doctor. If you stop taking it suddenly, your condition may worsen or you may have unwanted side effects.
Things you should be careful of
You should not drive or operate machinery on the day of treatment and for the next 24 hours because you may feel tired or dizzy after taking Biltricide.
Seek your doctor’s advice before taking any other medicines with Biltricide.
SIDE EFFECTS
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Biltricide.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Biltricide, whether or not they are mentioned below. You may need medical treatment in some cases.
Tell your doctor if you notice any of the following and they worry you:
- vomiting
- nausea
- diarrhoea
- loss of appetite
- stomach pains
- drowsiness, sleepiness
- general feeling of being unwell, unusual tiredness or weakness
- aching muscles, muscle tenderness or weakness
- headache
- dizziness, spinning sensation
- hives (redness and itchiness of the skin) or rash
- fever.
These are the more common side effects. It is often not clear, whether the side effects you experience are a result of taking Biltricide, caused by the death of the parasites or are symptoms caused by the parasite.
Tell your doctor immediately, or go to accident and emergency at your nearest hospital if you notice any of the following:
- signs of an allergic reaction e.g. rash, swelling of the face, lips, tongue or other parts of the body, shortness of breath, wheezing or trouble breathing
- itching
- seizures
- palpitations and or chest pain
- bloody diarrhoea.
These may be more serious side effects of Biltricide. You may need urgent medical attention. Serious side effects are uncommon.
Side effects seem to happen more often and be more noticeable if you are infected with a large number of the parasites. Some patients may experience mild liver problems.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Other side effects not listed above may also occur in some people.
AFTER TAKING BILTRICIDE
Storage
Keep your tablets in the bottle until it is time to take them. If you take the tablets out of the bottle they may not keep well.
Keep your tablets in a cool dry place where the temperature stays below 25°C.
Do not store it or any other medicine in the bathroom, near a sink, or on a window-sill.
Do not leave it in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Return any unused medicine to your pharmacist.
PRODUCT DESCRIPTION
What it looks like
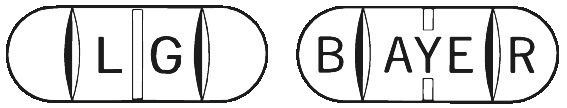
Biltricide is available in bottles of 8 tablets. They are white to pale yellow oblong tablets, with three score lines, marked with ‘BAYER’ on one side and ‘LG’ on the reverse.
Ingredients
Active Ingredient per tablet:
Biltricide - 600 mg praziquantel
Inactive ingredients:
- maize starch
- povidone
- sodium lauryl sulfate
- cellulose
- magnesium stearate
- hypromellose
- macrogol 4000
- titanium dioxide
Supplier
Made in Germany for:
Bayer Australia Ltd
ABN 22 000 138 714
875 Pacific Highway
Pymble NSW 2073
Bayer New Zealand Limited
3 Argus Place, Hillcrest
North Shore AUCKLAND 0627
Australian Registration Number
Biltricide - AUST R 18845
Date of preparation
July 2017
See TGA website (www.ebs.tga.gov.au) for latest Australian Consumer Medicine Information.
See MEDSAFE website (www.medsafe.govt.nz) for the latest New Zealand Consumer Medicine Information.
® Registered Trademark of Bayer AG, Germany
© Bayer Australia Ltd.
All rights reserved.

Published by MIMS December 2017

 Biltricide should be swallowed whole with a little liquid, preferably after meals.
Biltricide should be swallowed whole with a little liquid, preferably after meals. It is often not clear whether the complaints reported by patients or the undesirable effects reported by the physician are caused by praziquantel itself (1. direct relation), or may be considered to be an endogenous reaction to the death of the parasites produced by praziquantel (2. indirect relation), or are symptomatic observations of the infestation (3. no relation). It may be difficult to differentiate between the possible variations 1, 2 and 3.
It is often not clear whether the complaints reported by patients or the undesirable effects reported by the physician are caused by praziquantel itself (1. direct relation), or may be considered to be an endogenous reaction to the death of the parasites produced by praziquantel (2. indirect relation), or are symptomatic observations of the infestation (3. no relation). It may be difficult to differentiate between the possible variations 1, 2 and 3.
