1 Name of Medicine
Amlodipine besilate.
2 Qualitative and Quantitative Composition
Each Blooms Amlodipine 5 mg tablet contains 5 mg amlodipine.
Each Blooms Amlodipine 10 mg tablet contains 10 mg amlodipine.
For the full list of excipients, see Section 6.1 List of Excipients.
3 Pharmaceutical Form
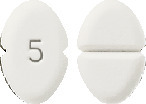
Blooms Amlodipine 5 mg tablet.
A white or almost white, oblong, bevelled tablet, scored on one side and coded "5" on the other.
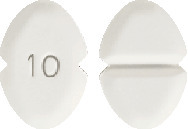
Blooms Amlodipine 10 mg tablet.
A white or almost white, oblong, bevelled tablet, scored on one side and coded "10" on the other.4.1 Therapeutic Indications
Hypertension.
First line treatment of hypertension. It can be used as the sole agent to control blood pressure in the majority of patients. Patients not adequately controlled on a single antihypertensive agent may benefit from the addition of amlodipine besilate, which has been used in combination with a thiazide diuretic, beta-adrenoreceptor blocking agent, or an angiotensin converting enzyme inhibitor.
Angina.
First line treatment of chronic stable angina. Amlodipine besilate may be used alone, as monotherapy, or in combination with other antianginal drugs.4.2 Dose and Method of Administration
Dosage.
For hypertension or angina, the usual initial dose is 2.5 to 5 mg once daily, which may be increased to a maximum dose of 10 mg depending on the individual patient's response.
Small, fragile or elderly individuals, or patients with hepatic insufficiency should be started on 2.5 mg once daily and this dose may be used when adding amlodipine besilate to other antihypertensive therapy.
Dosage should be adjusted according to each patient's need. In general, titration should proceed over 7 to 14 days so that the doctor can fully assess the patient's response to each dose level. Titration may proceed more rapidly, however, if clinically warranted, provided the patient is assessed frequently. (See Section 4.8 Adverse Effects (Undesirable Effects)).
Coadministration with other antihypertensive and/or antianginal drugs.
Amlodipine besilate has been safely administered with thiazides, angiotensin converting enzyme (ACE) inhibitors, beta-blockers, long acting nitrates, and/or sublingual nitroglycerine (glyceryl trinitrate).
No dose adjustment of amlodipine besilate is required upon concomitant administration of thiazide diuretics, beta-blockers, long acting nitrates and ACE inhibitors.4.3 Contraindications
Known hypersensitivity to amlodipine, other dihydropyridines, or any of the inactive ingredients.
4.4 Special Warnings and Precautions for Use
Increased angina.
Rarely patients, particularly those with severe obstructive coronary artery disease, have developed documented increased frequency, duration and/or severity of angina on starting calcium channel blocker therapy or at the time of dosage increase. The mechanism of this effect has not been elucidated.
Outflow obstruction (aortic stenosis).
Amlodipine besilate should be used with caution in the presence of a fixed left ventricular outflow obstruction (aortic stenosis).
Use in patients with congestive heart failure.
In general, calcium channel blockers should be used with caution in patients with heart failure. Amlodipine (5 to 10 mg/day) has been studied in a placebo controlled trial of 1,153 patients with NYHA class III or IV heart failure on stable doses of ACE inhibitor, digoxin and diuretics. Follow-up was at least six months, with a mean of about 14 months. There was no overall adverse effect on survival or cardiac morbidity (as defined by life threatening arrhythmia, acute myocardial infarction, or hospitalisation for worsened heart failure). Amlodipine besilate has been compared to placebo in four 8 to 12 week studies of patients with NYHA class II/III heart failure, involving a total of 697 patients. In these studies, there was no evidence of worsened heart failure based on measures of exercise tolerance, NYHA classification, symptoms, or LVEF.
Beta-blocker withdrawal.
Amlodipine besilate is not a beta-blocker and therefore provides no protection against the dangers of abrupt beta-blocker withdrawal; any such withdrawal should be by gradual reduction of the dose of beta-blocker.
Peripheral oedema.
Mild to moderate peripheral oedema was the most common adverse event in the clinical trials (see Section 4.8 Adverse Effects (Undesirable Effects)). The incidence of peripheral oedema was dose dependent and ranged in frequency from 3.0 to 10.8% in the 5 to 10 mg dose range. Care should be taken to differentiate this peripheral oedema from the effects of increasing left ventricular dysfunction.
Use in hepatic impairment.
There are no adequate studies in patients with liver dysfunction and dosage recommendations have not been established. In a small number of patients with mild to moderate hepatic impairment given single doses of amlodipine 5 mg, half-life has been prolonged. Worsening of liver function test values may occur. Amlodipine besilate should, therefore, be administered with caution in these patients and careful monitoring should be performed. A lower starting dose may be required (see Section 4.2 Dose and Method of Administration).
Use in renal impairment.
Amlodipine is extensively metabolised to inactive metabolites with 10% excreted as unchanged drug in the urine. Changes in amlodipine plasma concentrations are not correlated with the degree of renal impairment. Amlodipine besilate may be used at normal doses in patients with renal failure. Amlodipine is not dialysable.
Use in the elderly.
In elderly patients (≥ 65 years) clearance of amlodipine is decreased with a resulting increase in AUC. In clinical trials, the incidence of adverse events in elderly patients was approximately 6% higher than that of younger population (< 65 years). Adverse events include oedema, muscle cramps and dizziness. Amlodipine besilate should be used cautiously in elderly patients.
Paediatric use.
Amlodipine is not indicated in children. Safety and effectiveness have not been established in children.
Effects on laboratory tests.
No data available.4.5 Interactions with Other Medicines and Other Forms of Interactions
Amlodipine besilate has been safely administered with thiazide diuretics, beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, long acting nitrates, sublingual nitroglycerine (glyceryl trinitrate), non-steroidal anti-inflammatory drugs (NSAIDs), antibiotics and oral hypoglycaemic drugs.
Special studies have indicated that the co-administration of amlodipine besilate with digoxin did not change serum digoxin levels or digoxin renal clearance in healthy volunteers, and that co-administration of cimetidine did not alter the pharmacokinetics of amlodipine; and that co-administration with warfarin did not change the warfarin prothrombin response time.
In vitro data from studies with human plasma indicate that amlodipine has no effect on protein binding of the drugs tested (digoxin, phenytoin, warfarin or indomethacin).
Simvastatin.
Co-administration of multiple doses of amlodipine and simvastatin resulted in an increase in exposure to simvastatin compared to simvastatin alone. The Product Information for simvastatin should be reviewed for the appropriate dose of simvastatin when the patient is prescribed amlodipine concurrently.
Grapefruit juice.
Grapefruit juice is known to inhibit the cytochrome P450 system, thereby affecting the pharmacokinetics of drugs such as calcium channel blockers. Administration of amlodipine with grapefruit or grapefruit juice is not recommended as bioavailability may be increased in some patients resulting in increased blood pressure lowering effects.
CYP3A4 inhibitors.
With concomitant use with the CYP3A4 inhibitor erythromycin in young patients and diltiazem in elderly patients respectively, the plasma concentration of amlodipine increased. However, the clinical relevance of this finding is uncertain. It cannot be ruled out that strong inhibitors of CYP3A4 (i.e. ketoconazole, itraconazole, ritonavir) may increase the plasma concentrations of amlodipine to a greater extent than diltiazem. Amlodipine should be used with caution together with CYP3A4 inhibitors.
Clarithromycin.
Clarithromycin is an inhibitor of CYP3A4. There is an increased risk of hypotension in patients receiving clarithromycin with amlodipine. Close observation of patients is recommended when amlodipine is co-administered with clarithromycin.
CYP3A4 inducers.
There are no data available regarding the effect of CYP3A4 inducers on amlodipine. The concomitant use of CYP3A4 inducers (e.g. rifampicin, Hypericum perforatum [St John's wort]) may give a lower plasma concentration of amlodipine. Amlodipine should be used with caution together with CYP3A4 inducers.
Aluminium/ magnesium (antacid).
Coadministration of an aluminium/ magnesium antacid with a single dose of amlodipine had no significant effect on the pharmacokinetics of amlodipine.
Sildenafil.
A single 100 mg dose of sildenafil in 16 patients with essential hypertension had no effect on the pharmacokinetic parameters of amlodipine. When amlodipine and sildenafil were used in combination, each agent independently exerted its own blood pressure lowering effect.
Atorvastatin.
Coadministration of multiple 10 mg doses of amlodipine with atorvastatin 80 mg resulted in no significant change in the steady-state pharmacokinetic parameters of atorvastatin.
Ethanol (alcohol).
Single and multiple 10 mg doses of amlodipine had no significant effect on the pharmacokinetics of ethanol.
Ciclosporin.
No drug interaction studies have been conducted with ciclosporin and amlodipine in healthy volunteers or other populations, with the exception of renal transplant patients. Various studies in renal transplant patients report that co-administration of amlodipine with ciclosporin affects the trough concentrations of ciclosporin, and consideration should be given for monitoring ciclosporin levels in renal transplant patients on amlodipine.
Tacrolimus.
There is a risk of increased tacrolimus blood levels when co-administered with amlodipine. In order to avoid toxicity of tacrolimus, administration of amlodipine in a patient treated with tacrolimus requires monitoring of tacrolimus blood levels and dose adjustment of tacrolimus when appropriate.
Mechanistic target of rapamycin (mTOR) inhibitors.
mTOR inhibitors such as sirolimus, temsirolimus, and everolimus are CYP3A substrates. Amlodipine is a weak CYP3A inhibitor. Concomitant use of mTOR inhibitors and amlodipine may increase exposure of mTOR inhibitors.4.6 Fertility, Pregnancy and Lactation
Effects on fertility.
In animal studies, amlodipine did not affect fertility in rats at oral doses up to 18 mg/kg (base) and had no teratogenic effects in rats (18 mg/kg) or rabbits (10 mg/kg).
(Category C)
Calcium channel blockers carry the potential to produce fetal hypoxia associated with maternal hypotension. Accordingly, they should not be used in pregnant women unless the potential benefit outweighs the risk to the fetus.
Safety of amlodipine besilate in human pregnancy or lactation has not been established. Amlodipine (10 mg/kg as besilate salt, 7 mg/kg base) administered orally to rats at or near parturition induced a prolongation of gestation time, a prolonged duration of labour, an increase in the number of stillbirths and a decreased postnatal survival.
Experience in humans indicates that amlodipine is transferred into human breast milk. The median amlodipine concentration ratio of milk/plasma in 31 lactating women with pregnancy-induced hypertension was 0.85 following amlodipine administration at an initial dose of 5 mg once daily which was adjusted as needed (mean daily dose and body weight adjusted daily dose: 6 mg and 98.7 microgram/kg, respectively). The estimated daily dose of amlodipine in the infant via breast milk was 4.17 microgram/kg.
Breast-feeding should be discontinued during treatment with amlodipine besilate.4.7 Effects on Ability to Drive and Use Machines
Amlodipine can have minor or moderate influence on the ability to drive and use machines. If patients taking amlodipine suffer from dizziness, headache, fatigue or nausea the ability to react may be impaired.
4.8 Adverse Effects (Undesirable Effects)
Amlodipine besilate has been evaluated for safety in more than 11,000 patients in clinical trials worldwide.
In general, treatment with amlodipine besilate was well tolerated at doses up to 10 mg daily. Most adverse events reported during therapy with amlodipine besilate were of mild or moderate severity. In controlled clinical trials directly comparing amlodipine besilate (n = 1,730) in doses up to 10 mg to placebo (n = 1,250), discontinuation of amlodipine besilate due to adverse events was required in only about 1.5% of patients and was not significantly different from placebo (about 1%). Amlodipine besilate therapy has not been associated with clinically significant changes in routine laboratory tests. No clinically relevant changes were noted in serum potassium, serum glucose, total triglycerides, total cholesterol, high density lipoprotein (HDL) cholesterol, uric acid, blood urea nitrogen, creatinine or liver function tests.
The most common side effects are headache and oedema. The incidence (%) of side effects which occurred in a dose related manner are listed in Table 1.
 Other adverse experiences which were not clearly dose related but which were reported with an incidence greater than 1.0% in placebo controlled clinical trials include the following. See Table 2.
Other adverse experiences which were not clearly dose related but which were reported with an incidence greater than 1.0% in placebo controlled clinical trials include the following. See Table 2.
 The following events occurred in ≤ 1% but > 0.1% of patients in controlled clinical trials or under conditions of open trials or marketing experience where a causal relationship is uncertain; they are listed to alert the doctor to a possible relationship:
The following events occurred in ≤ 1% but > 0.1% of patients in controlled clinical trials or under conditions of open trials or marketing experience where a causal relationship is uncertain; they are listed to alert the doctor to a possible relationship:
Cardiac disorders.
Tachycardia, palpitations.
Vascular disorders.
Hot flushes, hypotension, peripheral ischaemia, postural hypotension, vasculitis.
Ear and labyrinth disorders.
Tinnitus, vertigo.
Nervous system disorders.
Hypoesthesia, paraesthesia, postural dizziness, syncope, tremor, peripheral neuropathy.
Eye disorders.
Abnormal vision, conjunctivitis, diplopia, eye pain.
Gastrointestinal disorders.
Anorexia, altered bowel habits, constipation, diarrhoea, dry mouth, dyspepsia*, dysphagia, flatulence, gingival hyperplasia, pancreatitis, vomiting.
General disorders and administration site conditions.
Asthenia*, chest pain, malaise, pain, rigors, thirst.
Immune system disorders.
Allergic reactions.
Musculoskeletal connective tissue and bone disorders.
Arthralgia, arthrosis, back pain, muscle cramps*, myalgia, ankle swelling.
Psychiatric disorders.
Abnormal dreams, anxiety, depersonalisation, depression, insomnia, mood changes, nervousness.
Respiratory, thoracic and mediastinal disorders.
Dyspnoea*, epistaxis.
Skin and subcutaneous tissue disorders.
Alopecia, angioedema, pruritus*, purpura, skin discolouration, hyperhidrosis, exanthema, rash*, rash erythematous, rash maculopapular, sweating increased.
Renal and urinary disorders.
Micturition disorder, micturition frequency, nocturia, increased urinary frequency.
Metabolism and nutritional disorders.
Hyperglycaemia, anorexia.
Blood and lymphatic system disorders.
Leucopenia, thrombocytopenia.
Reproductive system and breast disorder.
Gynaecomastia, sexual dysfunction (male* and female), impotence.
Investigations.
Weight increase, weight decrease.
* These events occurred in less than 1% of patients in placebo controlled trials, but the incidence of these side effects was between 1 and 2% in all multiple dose studies.
The following events occurred in ≤ 0.1% of patients: cardiac failure, pulse irregularity, extrasystoles, skin discolouration, urticaria, skin dryness, dermatitis, erythema multiforme, Stevens-Johnsons syndrome, photosensitivity, Quinke oedema, muscle weakness, twitching, ataxia, hypertonia, migraine, cold and clammy skin, apathy, agitation, amnesia, gastritis, increased appetite, loose stools, coughing, rhinitis, dysuria, polyuria, parosmia, taste perversion, abnormal visual accommodation, xerophthalmia and weight decrease.
As with other calcium channel blockers, the following adverse events have been rarely reported and cannot be distinguished from the natural history of the underlying disease: myocardial infarction, arrhythmia (including bradycardia, ventricular tachycardia and atrial fibrillation), chest pain and vasculitis.
There have been infrequent, post-marketing reports of hepatitis, jaundice and hepatic enzyme elevations (mostly consistent with cholestasis). Some cases severe enough to require hospitalisation have been reported in association with use of amlodipine. In many instances, causal association is uncertain.
There have been post-marketing reports of extrapyramidal disorder in association with use of amlodipine.
Amlodipine besilate has been used safely in patients with chronic obstructive pulmonary disease, well compensated congestive heart failure, peripheral vascular disease, diabetes mellitus and abnormal lipid profiles.
Reporting suspected adverse effects.
Reporting suspected adverse reactions after registration of the medicinal product is important. It allows continued monitoring of the benefit-risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions at http://www.tga.gov.au/reporting-problems.4.9 Overdose
Signs and symptoms.
Non-cardiogenic pulmonary oedema has rarely been reported as a consequence of amlodipine overdose that may manifest with a delayed onset (24-48 hours post-ingestion) and require ventilatory support. Early resuscitative measures (including fluid overload) to maintain perfusion and cardiac output may be precipitating factors.
Available data suggest that overdose might be expected to cause excessive peripheral vasodilation with marked hypotension and possibly a reflex tachycardia. Dysrhythmias may occur following overdose with any calcium antagonists. Hypotension and bradycardia are usually seen within 1 to 5 hours following overdose. Hypotension can persist for longer than 24 hours despite treatment. Cardiac rhythm disturbances have been noted to persist for up to 7 days. Marked and probably prolonged systemic hypotension up to and including shock with fatal outcome have been reported.
Reports of intentional overdosage include a patient who ingested 250 mg and was asymptomatic and was not hospitalised; another (120 mg) was hospitalised, underwent gastric lavage and remained normotensive; the third one (105 mg) was hospitalised and had hypotension (90/50 mmHg) which normalised following plasma expansion. Death resulted from a mixed overdose of 140 mg and 10 mefenamic acid capsules in a 15-year old girl and from a mixed overdose of amlodipine 70 mg and an unknown quantity of oxazepam in a 63-year old woman. A case of accidental drug overdose has been documented in a 19-month old male who ingested amlodipine besilate 30 mg (about 2 mg/kg). During the emergency room presentation, vital signs were stable with no evidence of hypotension, but a heart rate of 180 beats per minute.
Recommended treatment.
If massive overdose should occur, active cardiac and respiratory monitoring should be instituted. Frequent blood pressure measurements are essential. Should hypotension occur, cardiovascular support including elevation of the extremities and the judicious administration of fluids should be initiated. If hypotension remains unresponsive to these conservative measures, administration of vasopressors (such as phenylephrine) should be considered with attention to circulating volume and urine output. Intravenous calcium may help to reverse the effects of calcium entry blockade. Administration of activated charcoal to healthy volunteers immediately or up to two hours after ingestion of amlodipine 10 mg has been shown to significantly decrease amlodipine absorption. In patients who are not fully conscious or have impaired gag reflex, consideration should be given to administering activated charcoal via nasogastric tube once the airway is protected. Ipecac-emesis is not recommended since haemodynamic instability and CNS depression may rapidly develop. Since amlodipine is highly protein bound, dialysis is not likely to be of benefit.
For information on the management of overdose, contact the Poison Information Centre on 131126 (Australia).5 Pharmacological Properties
5.1 Pharmacodynamic Properties
Mechanism of action.
Amlodipine is a calcium ion influx inhibitor (slow channel blocker or calcium ion antagonist) and inhibits the transmembrane influx of calcium ions into cardiac and vascular smooth muscle.
Experimental data suggest that amlodipine binds to both dihydropyridine and nondihydropyridine binding sites. The contractile processes of cardiac muscle and vascular smooth muscle are dependent upon the movement of extracellular calcium ions into these cells through specific ion channels. Amlodipine inhibits calcium ion influx across cell membranes selectively, with a greater effect on vascular smooth muscle cells than on cardiac muscle cells. Negative inotropic effects can be detected in vitro but such effects have not been seen in intact animals at therapeutic doses. Serum calcium concentration is not affected by amlodipine. Within the physiological pH range, amlodipine is an ionised compound (pKa = 8.6), and its kinetic interaction with the calcium channel receptor is characterised by a gradual rate of association and dissociation with the receptor binding site, resulting in a gradual onset of effect.
Amlodipine is a peripheral arterial vasodilator that acts directly on vascular smooth muscle to cause a reduction in peripheral vascular resistance and reduction in blood pressure. The precise mechanism by which amlodipine relieves angina has not been fully determined but amlodipine reduces the total ischaemic burden by the following two actions.
Firstly, it dilates peripheral arterioles and thus reduces the total peripheral resistance (afterload) against which the heart works. Since the heart rate remains stable, this unloading of the heart reduces myocardial energy consumption and oxygen requirements.
Secondly, amlodipine has been shown to block constriction in main coronary arteries and coronary arterioles, induced by calcium, potassium, adrenaline, serotonin and thromboxane A2 analogue both in normal and in ischaemic regions.
Haemodynamics.
Following administration of therapeutic doses to patients with hypertension, amlodipine besilate produces vasodilation resulting in a reduction of supine and standing blood pressures. These decreases in blood pressure are not accompanied by a significant change in heart rate or plasma catecholamine levels with chronic dosing. Although the acute intravenous administration of amlodipine decreased arterial blood pressure and increased heart rate in haemodynamic studies of patients with chronic stable angina, chronic administration of oral amlodipine in clinical trials did not lead to clinically significant changes in heart rate or blood pressures in normotensive patients with angina.
With chronic once daily oral administration, antihypertensive effectiveness is maintained for at least 24 hours. Plasma concentrations correlate with effect in both young and elderly patients.
The magnitude of reduction in blood pressure with amlodipine besilate is also correlated with the height of pretreatment elevation; thus, individuals with moderate hypertension (diastolic pressure 105 to 114 mmHg) had about a 50% greater response than patients with mild hypertension (diastolic pressure 90 to 104 mmHg). Normotensive subjects experienced no clinically significant change in blood pressures (+1/-2 mmHg).
As with other calcium channel blockers, haemodynamic measurements of cardiac function at rest and during exercise (or pacing) in patients with normal ventricular function treated with amlodipine besilate have generally demonstrated a small increase in cardiac index without significant influence on dP/dt or on left ventricular end diastolic pressure or volume. In haemodynamic studies, amlodipine besilate has not been associated with a negative inotropic effect when administered in the therapeutic dose range to intact animals and humans, even when co-administered with beta-blockers to humans. Similar findings, however, have been observed in normal or well compensated patients with heart failure with agents possessing significant negative inotropic effects.
In hypertensive patients with normal renal function, therapeutic doses of amlodipine besilate resulted in a decrease in renal vascular resistance and an increase in glomerular filtration rate and effective renal plasma flow without change in filtration fraction or proteinuria.
Electrophysiological effects.
Amlodipine does not change sinoatrial nodal function or atrioventricular conduction in intact animals or humans. In patients with chronic stable angina, intravenous administration of amlodipine 10 mg and a further 10 mg after a 30 minute interval produced peripheral vasodilation and afterload reduction, but did not significantly alter atrial-His (A-H) and His-ventricular (H-V) conduction and sinus node recovery time after pacing. Similar results were obtained in patients receiving amlodipine besilate and concomitant beta-blockers. In clinical studies in which amlodipine besilate was administered in combination with beta-blockers to patients with either hypertension or angina, no adverse effects on electrocardiographic parameters were observed. In clinical trials with angina patients alone, amlodipine besilate therapy did not alter electrocardiographic intervals or produce higher degrees of atrioventricular blocks.
Effects in hypertension.
In patients with hypertension, once daily dosing provides clinically significant reductions of blood pressure in both the supine and standing positions throughout the 24 hour interval postdose. Due to the slow onset of action, acute hypotension is not a feature of amlodipine administration. The blood pressure effect is maintained over the 24 hour dosing interval, with little difference in peak and trough effect. Tolerance has not been demonstrated in patients studied for up to one year. Effects on diastolic pressure were similar in young and older patients. The effect on systolic pressure was greater in older patients, perhaps because of greater baseline systolic pressure.
Effects in chronic stable angina.
In patients with angina, once daily administration of amlodipine increases total exercise time to angina onset and total work time to 1 mm ST segment depression and decreases both angina attack frequency and nitroglycerine (glyceryl trinitrate) tablet consumption. The sustained efficacy of amlodipine besilate in angina patients has been demonstrated over long-term dosing. In patients with angina there were no clinically significant reductions in blood pressures (4/1 mmHg) or changes in heart rate (+0.3 beats per minute).
Other.
In clinical trials, amlodipine has shown no harmful effect on lipid levels. Dihydropyridine calcium channel blockers have not been associated with any adverse metabolic effects and are suitable for use in patients with asthma, diabetes and gout.
Clinical trials.
Studies in patients with congestive heart failure.
Amlodipine besilate has been compared to placebo in four 8 to 12 week studies of patients with New York Heart Association (NYHA) class II/III heart failure, involving a total of 697 patients. Although efficacy in regard to the primary and secondary endpoints was not demonstrated, there was no evidence of worsened heart failure based on measures of exercise tolerance, NYHA classification, symptoms, or LVEF. In a long-term (follow-up at least six months, mean 13.8 months) placebo controlled mortality/ morbidity study of amlodipine besilate 5 to 10 mg in 1,153 patients with NYHA classes III (n = 931) or IV (n = 222) heart failure on stable doses of diuretics, digoxin and ACE inhibitors, amlodipine besilate had no effect on the primary endpoint of the study which was the combined endpoint of all-cause mortality and cardiac morbidity (as defined by life threatening arrhythmia, acute myocardial infarction or hospitalisation for worsened heart failure), or on NYHA classification or symptoms of heart failure. Total combined all-cause mortality and cardiac morbidity events were 222/571 (39%) for patients on amlodipine besilate and 246/583 (42%) for patients on placebo: the cardiac morbid events represented about 25% of the endpoints in the study.
In this study, amlodipine was associated with increased reports of pulmonary oedema despite no significant difference in the incidence of worsening heart failure as compared to placebo.
5.2 Pharmacokinetic Properties
Absorption.
After oral administration of therapeutic doses, amlodipine is well absorbed with peak blood levels between 6 - 12 hours post-dose. This may reflect significant initial uptake by the liver, followed by a phase of redistribution. This interval is shorter (two to eight hours) in patients with hepatic insufficiency. Absolute bioavailability has been estimated to be between 64 and 90%. The bioavailability of amlodipine is not altered by the presence of food.
Distribution.
The volume of distribution is approximately 20 L/kg. In vitro studies have shown that approximately 97.5% of circulating amlodipine is bound to plasma proteins.
Metabolism.
Amlodipine is extensively metabolised by the liver to inactive metabolites.
Excretion.
10% of the parent compound and 60% of metabolites is excreted in the urine.
The terminal plasma elimination half-life is about 35 to 50 hours and is consistent with once daily dosing. Steady-state plasma levels are reached after seven to eight days of consecutive dosing.
Special populations.
Elderly ≥ 65 years.
In elderly hypertensive patients (mean age 69 years) there was a decrease in clearance of amlodipine from plasma as compared to young volunteers (mean age 36 years) with a resulting increase in the area under the curve (AUC) of about 60%.
5.3 Preclinical Safety Data
Genotoxicity.
No data available.
Carcinogenicity.
The carcinogenic potential of amlodipine has not been fully elucidated. Amlodipine did not induce any tumours when tested in rats at oral doses up to 2.5 mg/kg. This dose gave rise to plasma levels that are similar to those achieved clinically.6 Pharmaceutical Particulars
6.1 List of Excipients
Blooms Amlodipine tablets also contain sodium starch glycollate, calcium hydrogen phosphate, microcrystalline cellulose and magnesium stearate.
6.2 Incompatibilities
Incompatibilities were either not assessed or not identified as part of the registration of this medicine.
For information on interactions with other medicines and other forms of interactions, see Section 4.5 Interactions with Other Medicines and Other Forms of Interactions.
6.3 Shelf Life
In Australia, information on the shelf life can be found on the public summary of the Australian Register of Therapeutic Goods (ARTG). The expiry date can be found on the packaging.
6.4 Special Precautions for Storage
Store below 25°C. Protect from light.
6.5 Nature and Contents of Container
Blooms Amlodipine tablets are available in PA/Al/PVC/Al or PVC/Al blister packs of 30 tablets.
6.6 Special Precautions for Disposal
In Australia, any unused medicine or waste material should be disposed of by taking to your local pharmacy.
6.7 Physicochemical Properties
Amlodipine besilate is a white or almost white powder, slightly soluble in water, freely soluble in methanol, sparingly soluble in ethanol, slightly soluble in 2-propanol.
Chemical structure.
The chemical name of Amlodipine besilate is 3-ethyl 5-methyl-2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylate benzene sulfonate. Its empirical formula is C20H25ClN2O5.C6H6O3S (MW: 567.1) (free base 408.9) and its chemical structure:

CAS number.
111470-99-6 (amlodipine besilate).
88150-42-9 (amlodipine).7 Medicine Schedule (Poisons Standard)
S4 - Prescription Only Medicine.
Summary Table of Changes


 Other adverse experiences which were not clearly dose related but which were reported with an incidence greater than 1.0% in placebo controlled clinical trials include the following. See Table 2.
Other adverse experiences which were not clearly dose related but which were reported with an incidence greater than 1.0% in placebo controlled clinical trials include the following. See Table 2. The following events occurred in ≤ 1% but > 0.1% of patients in controlled clinical trials or under conditions of open trials or marketing experience where a causal relationship is uncertain; they are listed to alert the doctor to a possible relationship:
The following events occurred in ≤ 1% but > 0.1% of patients in controlled clinical trials or under conditions of open trials or marketing experience where a causal relationship is uncertain; they are listed to alert the doctor to a possible relationship:
