What is in this leaflet
This leaflet answers some common questions about Brintellix. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking Brintellix against the benefits they expect it will have for you.
If you have any concerns about taking this medicine ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What Brintellix is used for
Brintellix contains vortioxetine, which is used to treat major depression in adults.
Depression is longer lasting or more severe than the "low moods" everyone has from time to time due to the stress of everyday life. It is thought to be caused by a chemical imbalance in parts of the brain. This imbalance affects your whole body and can cause emotional, physical and cognitive symptoms such as feeling low in spirit, reduced ability to think or concentrate, or indecisiveness, loss of interest in activities, being unable to enjoy life, poor appetite or overeating, disturbed sleep, often waking up early, loss of sex drive, lack of energy and feeling guilty over nothing.
Brintellix is thought to work by its actions on multiple brain chemicals including serotonin, noradrenaline, dopamine, histamine and acetylcholine which are thought to be involved in controlling mood and related mental processes.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
Brintellix is available only with a doctor's prescription.
Brintellix is not addictive.
Brintellix should not be given to children under 18 years of age.
Before you take Brintellix
When you must not take it
Do not take Brintellix if you have an allergy to:
- vortioxetine
- any of the ingredients listed at the end of this leaflet
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin
Do not take Brintellix at the same time as the following other medicines:
- monoamine oxidase inhibitors (MAOIs), such as phenelzine, tranylcypromine, selegiline, rasagiline and moclobemide
- medicines which act like a monoamine oxidase inhibitor, such as the antibiotic linezolid
Taking Brintellix with MAOIs may cause a serious reaction including sudden changes in mental state, twitching, rapid heartbeat, high blood pressure, fever and diarrhoea.
If you have taken an MAOI, you will need to wait at least 14 days before starting Brintellix. If you have used moclobemide, one day must elapse after you stop taking moclobemide before you start taking Brintellix.
After stopping Brintellix, you must allow at least 14 days before taking any MAOI or moclobemide.
Do not take Brintellix after the expiry date (EXP) printed on the pack. The expiry date refers to the last day of the month. It may have no effect at all or, an entirely unexpected effect if you take it after the expiry date.
Do not take Brintellix if the packaging is torn or shows signs of having been tampered with.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you are pregnant or plan to become pregnant. Brintellix should not be used during pregnancy unless the benefit outweighs the risk. Your doctor can discuss with you the risks and benefits involved.
When taken during pregnancy, particularly in the last three months of pregnancy, medicines like Brintellix may increase the risk of a serious condition in babies called persistent pulmonary hypertension of the newborn (PPHN), making the baby breathe faster and appear bluish. These symptoms usually begin during the first 24 hours after the baby is born.
Other effects on your baby may include difficulty breathing, fits, body temperature changes, feeding difficulties, vomiting, stiff or floppy muscles, tremor, jitteriness, irritability, lethargy, constant crying, sleepiness or sleeping difficulties.
If your newborn baby has any of the above symptoms, you should contact your doctor immediately.
Tell your doctor if you are breast-feeding or planning to breast-feed It is recommended that you do not breast-feed while taking Brintellix, as it may be excreted in the milk.
Tell your doctor if you have, or have had, the following medical conditions:
- A tendency to bleed or bruise easily
- Low sodium levels in the blood
- Seizures or fits
- A history of suicide-related events or suicidal ideas
- A history, or family history, of mania or bipolar disorder (manic depression)
- Other diseases affecting the brain, including psychiatric conditions
- Other significant medical illnesses, such as unstable heart disease, stroke or severe liver or kidney disease
- Glaucoma, risk of glaucoma, or any condition affecting the fluid pressure in the eye. If your eyes become painful and you develop blurred vision during treatment, contact your doctor
When you are on antidepressant treatment, including vortioxetine, you may also experience feelings of aggression, agitation, anger and irritability. If this occurs, you should talk to your doctor.
Tell your doctor if are 65 years of age or older.
Tell your doctor if you are receiving electroconvulsive therapy.
Do not give Brintellix to a child or adolescent. There is no experience with the use of Brintellix in children or adolescents less than 18 years of age.
If you have not told your doctor about any of the above, tell them before you use Brintellix. Ask your doctor or pharmacist for advice before taking any medicine.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you buy without a prescription from a pharmacy, supermarket or health food shop.
Some medicines and Brintellix may interfere with each other. These include:
- Monoamine oxidase inhibitors (MAOIs), including selegiline, rasagiline, linezolid and moclobemide.
You must stop taking MAOIs at least two weeks before starting Brintellix. You must wait at least one day after finishing moclobemide before you start Brintellix.
You must stop taking Brintellix at least two weeks before you start taking an MAOI or moclobemide. - rifampicin, an antibiotic
- St. John's Wort, a herbal remedy
- sumatriptan (and similar medicines ending in 'triptan'), medicines used to treat migraine headaches
- lithium, used to treat mood swings and some types of depression
- tryptophan, an amino acid
- tramadol and similar medicines used to relieve pain
- mefloquine, an anti-malaria medicine
- bupropion, a medicine for nicotine dependence
- medicines known to cause low sodium level in the blood
- medicines used to thin the blood or known to prolong bleeding e.g. warfarin, dipyridamole, aspirin, and non-steroidal anti-inflammatory drugs (NSAIDs)
- antipsychotics, a class of medicines used to treat mental illness, e.g. risperidone, olanzapine, quetiapine
- tricyclic antidepressants, e.g. imipramine, desipramine
- any other medicines for depression, anxiety, panic disorder, obsessive-compulsive disorder or pre-menstrual dysphoric disorder
These medicines may be affected by Brintellix, or may affect how well it works. You may need to use different amounts of medicines, or take different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
If you have not told your doctor or pharmacist about any of these things, tell them before you take Brintellix.
If you are having a urine drug screen, taking Brintellix may cause positive results for methadone when some test methods are used, even though you may not be taking methadone. If this happens, a more specific test can be performed.
How to take Brintellix
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the label, ask your doctor or pharmacist for help.
How much to take
Your doctor will tell you how much Brintellix to take each day. Take the amount your doctor tells you to.
The usual dose for Brintellix in adults less than 65 years of age is 10 mg taken orally as one daily dose. The dose may be increased by your doctor to a maximum of 20 mg per day or lowered to a minimum of 5 mg per day.
For elderly people 65 years of age or older, the starting dose is 5 mg taken once daily.
Ask your doctor or pharmacist if you are unsure of the correct dose for you. They will tell you exactly how much to take.
Follow the instructions they give you. If you take the wrong dose, Brintellix may not work as well and your condition may not improve.
How to take it
Swallow the tablets whole with a full glass of water.
When to take it
Take Brintellix as a single dose either in the morning or in the evening.
Take your medicine at about the same time each day.
It does not matter if you take Brintellix before or after food.
How long to take it
Continue to take Brintellix even if it takes some time before you feel any improvement in your condition.
It may take two weeks, sometimes longer, before you feel any improvement.
Continue to take Brintellix for as long as your doctor recommends.
If your depression resolves while taking BRINTELLIX treatment should be continued for at least 6 months after you feel well again.
Your doctor will check your progress at regular intervals.
Do not change the dose or stop taking this medicine without first checking with your doctor.
If you forget to take it
Take the next dose at the usual time.
Do not take a double dose to make up for the dose that you missed. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or Poisons Information Centre (Telephone 13 11 26) for advice, or go to the Accident and Emergency department at your nearest hospital if you think that you or anyone else may have taken too much Brintellix. Take the Brintellix container with you when you go to the hospital. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
Keep the telephone number handy.
Some of the signs of an overdose could be dizziness, feeling sick (nausea), diarrhoea, stomach discomfort, itching on the whole body, sleepiness and flushing.
Following intake of dosage several times higher than the prescribed dose, fits (seizures) and a rare condition called serotonin syndrome have been reported.
While you are taking Brintellix
Things you must do
Tell your doctor immediately if you become pregnant while taking Brintellix.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking Brintellix.
Tell all doctors, dentists and pharmacists who are treating you that you are taking Brintellix.
Keep all of your doctor's appointments so that your progress can be checked.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking Brintellix. It may affect other medicines used during surgery.
If you have thoughts about killing yourself or other mental/mood changes, contact your doctor immediately or go to the nearest hospital for treatment. All mentions of suicide or violence must be taken seriously.
Occasionally, the symptoms of depression may include thoughts of suicide or self-harm. It is possible that these symptoms continue or get worse until the full antidepressant effect of the medicine becomes apparent.
This is more likely to occur:
- if you are a young adult, i.e. 18 to 24 years of age; or
- you have previously had thoughts about killing or harming yourself
You may find it helpful to tell a relative or close friend that you are depressed and ask them to read this leaflet. You might ask them to tell you if they think your depression is getting worse, or if they are worried about changes in your behaviour.
Patients and care givers should pay attention for any of the following warning signs of suicide-related behaviour while taking Brintellix:
- worsening of depression
- thoughts or talk of death or suicide
- thoughts or talk of self-harm or harm to others
- any recent attempts of self-harm
- increase in aggressive behaviour, irritability, agitation or any other unusual changes in behaviour or mood.
Things you must not do
Do not take Brintellix to treat any other complaints unless your doctor tells you to.
Do not give Brintellix to anyone else, even if they have the same condition as you.
Do not stop taking Brintellix, or lower the dosage, without checking with your doctor. If you stop taking it suddenly, or reduce the amount you take, your condition may worsen.
Things to be careful of
Be careful driving or operating machinery until you know how Brintellix affects you.
Avoid alcohol while you are taking Brintellix.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Brintellix. Brintellix helps most people with depression, but it may have unwanted side effects in some people.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if you notice any of the following side effects and they worry you:
- feeling sick (nausea)
- diarrhoea or constipation
- vomiting
- influenza type symptoms
- decreased appetite
- sedation
- itching on the whole body
The above list includes the more common side effects of your medicine.
Side effects are generally mild to moderate and occur within the first two weeks of treatment. They are usually temporary and generally do not require treatment cessation.
Tell your doctor as soon as possible if you notice any of the following:
- mania* (symptoms include mood of excitement, over-activity and uninhibited behaviour)
- low sodium levels in the blood* (symptoms include feeling tired, weak and sick with weak muscles or feeling confused)
- bleeding tendency* (e.g. bruising)
- enlarged pupils (mydriasis), which can increase the risk of glaucoma*
- agitation and aggression
The above list includes serious side effects that may require medical attention.
Tell your doctor immediately, or go to Accident and Emergency at your nearest hospital, if you notice any of the following:
- Thoughts of harming yourself or thoughts of suicide, see also section "Things you must do"*
- serious allergic reaction
(symptoms of an allergic reaction may include swelling of the face, lips, mouth or throat which may cause difficulty in swallowing or breathing, or rash, itching or hives) - high fever, agitation, confusion, trembling and abrupt contractions of muscles
(these symptoms may be signs of a rare condition called serotonin syndrome)* - seizures*
- bleeding* (e.g. vomiting of blood, traces of blood in your stools or your stools are dark in colour)
The above list includes very serious side effects. You may need urgent medical attention or hospitalisation.
* The side effects marked with an asterisk (*) are a number of rare side effects that are known to occur with medicines that work in a similar way to Brintellix.
Tell your doctor or pharmacist if you notice anything else that is making you feel unwell. Other side effects not listed above may also occur in some people.
After taking Brintellix
Storage
Keep Brintellix in the blister pack until it is time to take them. If you take the tablets out of the box or blister pack they may not keep well.
Keep Brintellix in a cool dry place where the temperature stays below 30°C.
Do not store Brintellix or any other medicine in the bathroom or near a sink or stove. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep Brintellix where young children cannot reach it. A locked cupboard at least one-and-a-half metres above ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine you may have left over.
Brintellix description
What Brintellix tablets look like
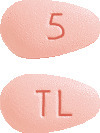
- Brintellix 5 mg tablets are pink, almond-shaped, biconvex film-coated tablet engraved with "TL" on one side and "5" on the other side.
- Brintellix 10 mg tablets are yellow, almond-shaped, biconvex film-coated tablet engraved with "TL" on one side and "10" on the other side.
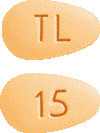
- Brintellix 15 mg tablets are orange, almond-shaped, biconvex film-coated tablet engraved with "TL" on one side and "15" on the other side.
- Brintellix 20 mg tablets are red, almond-shaped, biconvex film-coated tablet engraved with "TL" on one side and "20" on the other side.
Brintellix is available in blister packs of 28 tablets.
Ingredients
Active ingredients
- Brintellix 5 mg tablets contain 5 mg vortioxetine (as hydrobromide) per tablet
- Brintellix 10 mg tablets contain 10 mg vortioxetine (as hydrobromide) per tablet
- Brintellix 15 mg tablets contain 15 mg vortioxetine (as hydrobromide) per tablet
- Brintellix 20 mg tablets contain 20 mg vortioxetine (as hydrobromide) per tablet
Brintellix also contains:
- Mannitol
- Microcrystalline cellulose
- Hyprolose
- Sodium starch glycollate type A
- Magnesium stearate
- Hypromellose
- Titanium dioxide
- Macrogol 400
- Iron oxide red CI 77491 (5, 15 and 20 mg tablets)
- Iron oxide yellow CI 77492 (10 and 15 mg tablets)
Brintellix does not contain lactose, gluten, sucrose, tartrazine or any other azo dyes.
Manufacturer and distributor
Brintellix is made by H. Lundbeck A/S, Denmark.
Distributed in Australia by:
Lundbeck Australia Pty Ltd
1 Innovation Road
North Ryde NSW 2113
Ph: 02 8669 1000
Distributed in New Zealand by:
Pharmacy Retailing t/a Healthcare Logistics, 58 Richard Pearse Drive, Mangere, Auckland 2022 Ph: 0800 540 555
This leaflet was prepared on
18 October 2021
Australian Registration Numbers are:
5 mg tablet: AUST R 203986
10 mg tablet: AUST R 203955
15 mg tablet: AUST R 203981
20 mg tablet: AUST R 203970
New Zealand Registration Numbers are:
5 mg tablet: TT50-10588
10 mg tablet: TT50-10588a
15 mg tablet: TT50-10588b
20 mg tablet: TT50-10588c
Brintellix is a registered trade mark of H. Lundbeck A/S.
Copyright© 2014 H. Lundbeck A/S
AU-6594-CMI
Published by MIMS December 2021

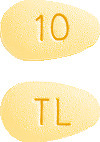


 The effect of vortioxetine on sexual function was further evaluated in an 8-week, double-blind, flexible-dose, comparative study (n=424) versus escitalopram in patients treated for at least 6 weeks with an SSRI (citalopram, paroxetine, or sertraline), with a low level of depressive symptoms (baseline CGI-S ≤ 3) and TESD induced by the prior SSRI treatment. Vortioxetine 10-20 mg/day had statistically significantly less TESD than escitalopram 10-20 mg/day as measured by change in the CSFQ-14 total score (2.2 points, p=0.013) at week 8. The proportion of responders was not significantly different in the vortioxetine group (162 (74.7%)) compared with the escitalopram group (137 (66.2%)) at week 8 (OR 1.5 p=0.057). The antidepressant effect was maintained in both treatment groups. See Figure 1.
The effect of vortioxetine on sexual function was further evaluated in an 8-week, double-blind, flexible-dose, comparative study (n=424) versus escitalopram in patients treated for at least 6 weeks with an SSRI (citalopram, paroxetine, or sertraline), with a low level of depressive symptoms (baseline CGI-S ≤ 3) and TESD induced by the prior SSRI treatment. Vortioxetine 10-20 mg/day had statistically significantly less TESD than escitalopram 10-20 mg/day as measured by change in the CSFQ-14 total score (2.2 points, p=0.013) at week 8. The proportion of responders was not significantly different in the vortioxetine group (162 (74.7%)) compared with the escitalopram group (137 (66.2%)) at week 8 (OR 1.5 p=0.057). The antidepressant effect was maintained in both treatment groups. See Figure 1. In a 5-week, double-blind, placebo- and paroxetine-controlled study in healthy subjects with normal sexual functioning, without the confounding effect of depression, vortioxetine 10 mg/day (2.74 points, p=0.009) was statistically significantly better than paroxetine 20 mg/day as measured by change in the CSFQ-14 total score, whereas vortioxetine 20 mg/day was not (1.05 points, p=0.303). Neither vortioxetine 10 mg/day nor 20 mg/day were significantly worse than placebo. See Figure 2.
In a 5-week, double-blind, placebo- and paroxetine-controlled study in healthy subjects with normal sexual functioning, without the confounding effect of depression, vortioxetine 10 mg/day (2.74 points, p=0.009) was statistically significantly better than paroxetine 20 mg/day as measured by change in the CSFQ-14 total score, whereas vortioxetine 20 mg/day was not (1.05 points, p=0.303). Neither vortioxetine 10 mg/day nor 20 mg/day were significantly worse than placebo. See Figure 2.




