1 Name of Medicine
Candesartan cilexetil.
2 Qualitative and Quantitative Composition
Each BTC Candesartan 4 mg tablet contains 4 mg candesartan cilexetil.
Each BTC Candesartan 8 mg tablet contains 8 mg candesartan cilexetil.
Each BTC Candesartan 16 mg tablet contains 16 mg candesartan cilexetil.
Each BTC Candesartan 32 mg tablet contains 32 mg candesartan cilexetil.
Excipient with known effect.
Sugars as lactose.
For the full list of excipients, see Section 6.1 List of Excipients.3 Pharmaceutical Form
BTC Candesartan 4 mg tablets are white, round biconvex tablets, debossed with 4 on one side and scored on the other.
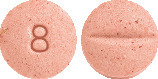
BTC Candesartan 8 mg tablets are pink, mottled, round biconvex tablets, debossed with 8 on one side and scored on the other.
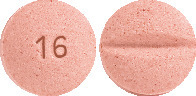
BTC Candesartan 16 mg tablets are pink, mottled, round biconvex tablets, debossed with 16 on one side and scored on the other.
BTC Candesartan 32 mg tablets are pink, mottled, round biconvex tablets, debossed with 32 on one side and scored on the other.
4.1 Therapeutic Indications
Treatment of hypertension.
Treatment of patients with heart failure and impaired left ventricular systolic function (left ventricular ejection fraction ≤ 40%) as add on therapy to angiotensin converting enzyme (ACE) inhibitors or when ACE inhibitors are not tolerated.
4.2 Dose and Method of Administration
Dosage.
BTC Candesartan should be taken once daily with or without food.
Hypertension.
The recommended maintenance dose of BTC Candesartan is 8 or 16 mg once daily. The maximal antihypertensive effect is attained within four weeks following initiation of treatment. For those patients who start on 8 mg and require further blood pressure reduction, a dose increase to 16 mg is recommended. An initial dose of 16 mg is also well tolerated. Some patients may receive an additional benefit by increasing the dose to 32 mg once daily.
In patients with less than optimal blood pressure reduction on BTC Candesartan, combination with a thiazide diuretic is recommended.
Heart failure.
The usual recommended initial dose of BTC Candesartan is 4 mg once daily. Up-titration to the target dose of 32 mg once daily or the highest tolerated dose is performed by doubling the dose at intervals of at least 2 weeks (see Section 4.4 Special Warnings and Precautions for Use).
Dosage adjustment.
Renal impairment.
No initial dosage adjustment is necessary in patients with mild to moderate impaired renal function (i.e. creatinine clearance 30 - 80 mL/minute/1.73 m2 body surface area (BSA)). In patients with severely impaired renal function (i.e. creatinine clearance < 30 mL/minute/1.73 m2 BSA), including patients on haemodialysis, a lower initial dose of 4 mg should be considered.
Hepatic impairment.
Dose titration is recommended in patients with mild to moderate chronic liver disease, and a lower initial dose of 4 mg should be considered.
BTC Candesartan should not be used in patients with severe hepatic impairment and/or cholestasis (see Section 4.3 Contraindications).
Elderly.
An initial dose of 8 mg is recommended.
Paediatrics.
The safety and efficacy of BTC Candesartan have not been established in children.
Concomitant therapy.
BTC Candesartan can be administered with other heart failure treatment, including ACE inhibitors, beta-blockers, diuretics and digitalis or a combination of these medicines (see Section 4.4 Special Warnings and Precautions for Use; Section 5.1 Pharmacodynamic Properties).4.3 Contraindications
Hypersensitivity to any component of BTC Candesartan.
Pregnancy and lactation (see Section 4.6 Fertility, Pregnancy and Lactation, Use in pregnancy).
Severe hepatic impairment and/or cholestasis.
The use of candesartan in combination aliskiren-containing medicines in patients with diabetes mellitus (type I or II) or with moderate to severe renal impairment (GFR < 60 mL/min/1.73 m2).
4.4 Special Warnings and Precautions for Use
In patients whose vascular tone and renal function depend predominantly on the activity of the renin-angiotensin-aldosterone-system (RAAS) (e.g. patients with severe congestive heart failure or underlying renal disease, including renal artery stenosis), treatment with medicines that affect this system has been associated with acute hypotension, azotaemia, oliguria or, rarely, acute renal failure. As with any antihypertensive agent, excessive blood pressure decrease in patients with ischaemic heart disease or atherosclerotic cerebrovascular disease could result in a myocardial infarction or stroke.
Kidney transplantation.
There is limited clinical experience regarding candesartan use in patients who have undergone renal transplant.
Renal artery stenosis.
Other medicines that affect the RAAS, i.e. ACE inhibitors, may increase blood urea and serum creatinine in patients with bilateral renal artery stenosis or stenosis of the artery to a solitary kidney. A similar effect may be anticipated with angiotensin II receptor antagonists (AIIRAs).
Aortic and mitral valve stenosis or obstructive hypertrophic cardiomyopathy.
As with other vasodilators, special caution is indicated in patients suffering from haemodynamically relevant aortic or mitral valve stenosis, or obstructive hypertrophic cardiomyopathy.
Primary hyperaldosteronism.
Patients with primary hyperaldosteronism will not generally respond to antihypertensive medicines acting through inhibition of the RAAS. Therefore, the use of candesartan in these patients is not recommended.
Hypotension.
Hypotension may occur during treatment with candesartan in heart failure patients. As described for other agents acting on the RAAS, it may also occur in hypertensive patients with intravascular volume depletion. Caution should be observed when initiating therapy and correction of hypovolaemia should be attempted.
Dual blockade of the RAAS.
There is evidence that the concomitant use of ACE-inhibitors, AIIRAs or aliskiren increases the risk of hypotension, hyperkalaemia and decreased renal function (including acute renal failure). Dual blockade of the RAAS through the combined use of candesartan with and ACE-inhibitor or aliskiren is therefore not recommended (see Section 4.5 Interactions with Other Medicines and Other Forms of Interactions).
If dual blockade therapy is considered necessary, this should only occur under specialist supervision and subject to frequent close monitoring of renal function, electrolytes and blood pressure.
ACE-inhibitors and angiotensin II receptor blockers should not be used concomitantly in patients with diabetic nephropathy. The use of candesartan with aliskiren is contraindicated in patients with diabetes mellitus (type I or II) or moderate to severe renal impairment (GFR < 60 mL/min/1.73 m2) (see Section 4.3 Contraindications).
Use in heart failure.
Triple combination of candesartan with an ACE-inhibitor and a mineralocorticoid receptor antagonist used in heart failure is also not recommended. Use of these combinations should be under specialist supervision and subject to frequent close monitoring of renal function, electrolytes and blood pressure.
Hyperkalaemia.
Based on experience with the use of other medicines that affect the RAAS, concomitant use of candesartan with potassium-sparing diuretics, potassium supplements, salt substitutes containing potassium, or other medicines that may increase potassium levels (e.g. heparin, trimethoprim/sulfamethoxazole) may lead to increases in serum potassium in hypertensive patients. In heart failure patients treated with candesartan, hyperkalaemia may occur. During treatment with candesartan in patients with heart failure, periodic monitoring of serum potassium is recommended, especially when taken concomitantly with ACE inhibitors and potassium-sparing diuretics such as spironolactone.
Anaesthesia and surgery.
Hypotension may occur during anaesthesia and surgery in patients treated with AIIRAs due to blockade of the renin-angiotensin system (RAS). Very rarely, hypotension may be severe such that it may warrant the use of intravenous fluids and/or vasopressors.
Combination use of ACE inhibitors or angiotensin receptor antagonists, anti-inflammatory drugs and thiazide diuretics.
The use of an ACE inhibiting medicine (ACE inhibitor or angiotensin receptor antagonist), an anti-inflammatory drug (NSAID or COX-2 inhibitor) and a thiazide diuretic at the same time increases the risk of renal impairment. This includes use in fixed combination products containing more than one class of medicine. Combined use of these medications should be accompanied by increased monitoring of serum creatinine, particularly at the institution of the combination. The combination of medicines from these three classes should be used with caution particularly in elderly patients or those with pre-existing renal impairment.
Use in hepatic impairment.
There is limited clinical experience in patients with severe hepatic impairment and/or cholestasis. Use in patients with severe hepatic impairment is contraindicated. Caution is advised in patients with mild to moderate hepatic impairment. There have been reports of clinically significant liver disease occurring with other AIIRAs. No such cases have been reported to date with candesartan.
Use in renal impairment.
As with other agents inhibiting the RAAS, changes in renal function may be anticipated in susceptible patients treated with candesartan. When candesartan is used in hypertensive patients with severe renal impairment, periodic monitoring of serum potassium and creatinine levels should be considered. There is very limited experience in patients with very severe or end-stage renal impairment (i.e. creatinine clearance < 15 mL/minute/1.73 m2 BSA). Evaluation of patients with heart failure should include periodic assessments of renal function. During dose titration of candesartan, monitoring of serum creatinine and potassium is recommended.
Haemodialysis.
During dialysis, the blood pressure may be particularly sensitive to AT1-receptor blockade as a result of reduced plasma volume and activation of the RAAS. Therefore, candesartan should be carefully titrated with thorough monitoring of blood pressure in patients on haemodialysis (see Section 4.2 Dose and Method of Administration).
Use in the elderly.
No data available.
Paediatric use.
The safety and efficacy of candesartan cilexetil have not been established in children.
Effects on laboratory tests.
In general, there were no clinically important effects of candesartan cilexetil on routine laboratory variables. As for other inhibitors of the RAAS, small decreases in haemoglobin have been seen. Increases in creatinine, urea or potassium and decreases in sodium have been observed. In clinical trials, elevations of alanine aminotransferase (ALT) occurred in 1.3% of candesartan treated patients and 0.5% of those treated with placebo. The incidence of aspartate aminotransferase (AST) elevation was 0.4% with candesartan and 0% with placebo. No routine monitoring of laboratory variables is usually necessary for patients receiving candesartan cilexetil. However, in patients with severe renal impairment, periodic monitoring of serum potassium and creatinine levels should be considered.4.5 Interactions with Other Medicines and Other Forms of Interactions
Dual blockade of the RAAS.
The combination of candesartan with aliskiren-containing medicine is contraindicated in patients with diabetes mellitus (type I or II) or moderate to severe renal impairment (GFR < 60 mL/min/1.7 m2) and is not recommended in other patients. Clinical trial data has shown that dual blockade of the RAAS through the combined use of ACE-inhibitors, angiotensin II receptor blocker or aliskiren is associated with a higher frequency of adverse events such as hypotension, hyperkalaemia and decreased renal function (including acute renal failure) compared to the use of a single RAAS-acting agent. (See Section 4.3 Contraindications; Section 4.4 Special Warnings and Precautions for Use.)
Food.
Food increases the rate of absorption of candesartan; however, the extent of absorption of candesartan is not affected by food.
Lithium.
Reversible increases in serum lithium concentrations and toxicity have been reported during concomitant administration of lithium with ACE inhibitors. A similar effect may occur with AIIRAs and careful monitoring of serum lithium levels is recommended during concomitant use.
Other medicines.
Compounds which have been investigated in clinical pharmacokinetic studies include hydrochlorothiazide, warfarin, digoxin, oral contraceptives (i.e. ethinyloestradiol/ levonorgestrel), glibenclamide, nifedipine and enalapril. No pharmacokinetic interactions of clinical significance were identified in these studies.
The antihypertensive effect of AIIRAs, including candesartan, may be attenuated by NSAIDs, including COX-2 inhibitors and acetylsalicylic acid.
As with ACE inhibitors, concomitant use of AIIRAs and NSAIDs may lead to an increased risk of worsening of renal function, including possible acute renal failure, and an increase in serum potassium, especially in patients with poor pre-existing renal function. The combination should be administered with caution, especially in older patients and in volume depleted patients. Patients should be adequately hydrated and consideration should be given to monitoring renal function after initiation of concomitant therapy and periodically thereafter.
Candesartan is eliminated only to a minor extent by hepatic metabolism (CYP2C9). Available interaction studies indicate no effect on CYP2C9 and CYP3A4 but the effect on other cytochrome P450 isoenzymes is presently unknown.
Candesartan may be administered with other antihypertensive agents.4.6 Fertility, Pregnancy and Lactation
Effects on fertility.
Candesartan cilexetil had no adverse effects on the reproductive performance of male or female rats at oral doses up to 300 mg/kg/day.
(Category D)
The use of candesartan is contraindicated during pregnancy (see Section 4.3 Contraindications). Patients receiving candesartan should be made aware of that before contemplating a possibility of becoming pregnant so that they can discuss appropriate options with their treating physician. When pregnancy is diagnosed, treatment with candesartan must be stopped immediately and if appropriate, alternative therapy should be started.
Drugs that act on the RAS can cause fetal and neonatal morbidity and death when administered to pregnant women. Exposure to angiotensin II receptor antagonist therapy is known to induce human fetotoxicity (decreased renal function, oligohydramnios, skull ossification retardation) and neonatal toxicity (renal failure, hypotension, hyperkalaemia).
It is not known whether candesartan is excreted in human milk. However, candesartan is excreted in the milk of lactating rats. Because of the potential for adverse effects on the nursing infant, breastfeeding should be discontinued if the use of candesartan is considered essential.4.7 Effects on Ability to Drive and Use Machines
When driving vehicles or operating machines, it should be taken into account that dizziness or weariness may occur during treatment.
4.8 Adverse Effects (Undesirable Effects)
Hypertension.
Candesartan cilexetil was well tolerated in clinical studies showing an adverse event profile comparable to that of placebo. Generally, adverse events were mild and transient. The overall incidence of adverse effects showed no association with dose, age or gender. Withdrawals from treatment due to adverse events were similar with candesartan cilexetil (3.1%) and placebo (3.2%).
Information on adverse events was obtained from 39 phase I to phase III clinical studies, involving a total of 5,464 subjects. Candesartan cilexetil was administered as monotherapy or combination therapy to 2,061 hypertensive patients. The crude frequency of the most commonly occurring adverse events, irrespective of causality, reported for those patients and the 573 placebo comparators are given in Table 1.

Heart failure.
The adverse experience profile of candesartan cilexetil in heart failure patients was consistent with the pharmacology of the medicine and the health status of the patients. In the CHARM clinical program, comparing candesartan cilexetil in doses up to 32 mg (n = 3,803) to placebo (n = 3,796), 21.0% of the candesartan cilexetil group and 16.1% of the placebo group discontinued treatment because of adverse events.
Adverse reactions commonly (≥ 1/100, < 1/10) seen were as follows:
Vascular disorders.
Hypotension.
Metabolism and nutrition disorders.
Hyperkalaemia.
Renal and urinary disorders.
Renal impairment.
Laboratory findings.
Increases in creatinine, urea and potassium. Periodic monitoring of serum creatinine and potassium is recommended (see Section 4.4 Special Warnings and Precautions for Use).
Postmarketing.
The following adverse reactions have been reported very rarely (< 0.01%) in postmarketing experience.
Blood and lymphatic system disorders.
Leucopenia, neutropenia and agranulocytosis.
Metabolism and nutrition disorders.
Hyperkalaemia, hyponatraemia.
Hepatobiliary disorders.
Increased liver enzymes, abnormal hepatic function or hepatitis.
Skin and subcutaneous tissue disorders.
Angioedema, rash, urticaria, pruritus.
Musculoskeletal, connective tissue and bone disorders.
Back pain, myalgia.
Renal and urinary disorders.
Renal impairment, including renal failure in susceptible patients (see Section 4.4 Special Warnings and Precautions for Use, Use in renal impairment).
Rare reports of rhabdomyolysis have been reported in patients receiving angiotensin II receptor blockers.
Although causality to candesartan has not been established, the following neuropsychiatric and cardiovascular adverse reactions have been very rarely reported during postmarketing surveillance. These were agitation, anxiety, depression, insomnia, somnolence, nervousness, nightmare, sleep disorder and palpitations.
Reporting suspected adverse effects.
Reporting suspected adverse reactions after registration of the medicinal product is important.
It allows continued monitoring of the benefit-risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions at www.tga.gov.au/reporting-problems.4.9 Overdose
Symptoms.
Based on pharmacological considerations, the main manifestation of an overdose is likely to be symptomatic hypotension and dizziness. In single case reports of overdose (up to 672 mg candesartan cilexetil), patient recovery was uneventful.
Treatment.
If symptomatic hypotension should occur, symptomatic treatment should be instituted and vital signs monitored. The patients should be placed supine with the legs elevated. If this is not sufficient, plasma volume should be increased by the infusion of, e.g. isotonic saline solution. Sympathomimetic medicines may be administered if the above-mentioned measures are not sufficient.
Candesartan is not removed by haemodialysis.
For information on the management of overdose, contact the Poison Information Centre on 131126 (Australia).5 Pharmacological Properties
5.1 Pharmacodynamic Properties
Mechanism of action.
Candesartan cilexetil is an AIIRA.
Angiotensin II is the primary vasoactive hormone of the RAAS and plays a significant role in the pathophysiology of hypertension, heart failure and other cardiovascular disorders. It also has an important role in the pathogenesis of end organ hypertrophy and damage. The major physiological effects of angiotensin II, such as vasoconstriction, aldosterone stimulation, regulation of salt and water homeostasis and stimulation of cell growth, are mediated via the type 1 (AT1) receptor.
Candesartan cilexetil is a prodrug suitable for oral use. It is rapidly converted to the active drug, candesartan, by ester hydrolysis during absorption from the gastrointestinal tract. Candesartan is an AIIRA, selective for AT1 receptors, with tight binding to and slow dissociation from the receptor. It has no agonist activity.
Candesartan does not inhibit ACE, which converts angiotensin I to angiotensin II and degrades bradykinin. Since there is no effect on ACE and no potentiation of bradykinin or substance P, AIIRAs are unlikely to be associated with cough. This has been confirmed in controlled clinical studies with candesartan cilexetil. Candesartan does not bind to or block other hormone receptors or ion channels known to be important in cardiovascular regulation.
In hypertension, candesartan cilexetil causes a dose dependent, long lasting reduction in arterial blood pressure. The antihypertensive action is due to decreased systemic peripheral resistance, while heart rate, stroke volume and cardiac output are not affected. There is no indication of serious or exaggerated first dose hypotension or rebound effect after cessation of treatment.
Candesartan cilexetil is effective in hypertension. After administration of a single dose, onset of antihypertensive effect generally occurs within two hours. With continuous treatment, the maximum reduction in blood pressure with any dose is generally attained within four weeks and is sustained during long-term treatment. It provides effective and smooth blood pressure reduction over the 24-hour dosing interval, with a trough/ peak ratio confirming once daily dosing.
Candesartan can be used as monotherapy or in combination with other antihypertensive medicines, such as thiazide diuretics, calcium antagonists and lisinopril, for improved blood pressure control. Age and gender have no influence on the efficacy of candesartan cilexetil.
Candesartan cilexetil has favourable renal haemodynamic effects. It increases renal blood flow and maintains or increases glomerular filtration rate while renal vascular resistance and filtration fraction are reduced. Candesartan cilexetil reduces urinary protein excretion in hypertensive patients with microalbuminuria or nephropathy of different aetiology. Candesartan cilexetil has no adverse effect on blood glucose or lipid profile.
In a variety of preclinical safety studies conducted in several species, expected exaggerated pharmacological effects (e.g. renal changes leading to juxtaglomerular cell hypertrophy, adrenal gland zona glomerulosa atrophy and reduced heart weight related to reduced afterload), due to modification of the RAAS homeostasis, have been observed. The incidence and severity of the effects induced were dose and time related and have been shown to be reversible in adult animals. Fetotoxicity has been observed in late pregnancy (see Section 4.6 Fertility, Pregnancy and Lactation, Use in pregnancy, Use in lactation).
Clinical trials.
Hypertension. The Candesartan and Lisinopril Microalbuminuria (CALM) study was a 24 week double blind, parallel group trial (n = 199) to evaluate the effects of candesartan and lisinopril alone and in combination on urinary albumin excretion (UAE) in patients with type 2 diabetes mellitus, hypertension and microalbuminuria. Patients were randomly allocated to four treatment regimens: 1) 24 weeks of candesartan monotherapy (one-third of the patients); 2) 24 weeks of lisinopril monotherapy (one-third of the patients); 3) 12 weeks of candesartan monotherapy, followed by 12 weeks of candesartan + lisinopril combination therapy (one-sixth of the patients); and 4) 12 weeks of lisinopril monotherapy, followed by 12 weeks of lisinopril and candesartan combination therapy (one-sixth of the patients). Thus, after 12 weeks, half of the patients were treated with candesartan monotherapy (n = 99) and half with lisinopril monotherapy (n = 98). After 24 weeks, one-third of the patients still in the study were on candesartan monotherapy (n = 49), one-third on lisinopril monotherapy (n = 46), and one-third on combination therapy (candesartan and lisinopril (n = 25), lisinopril and candesartan (n = 24). (See Table 2.)
Significant reduction in urinary albumin/ creatinine ratio (UACR) in both monotherapy treatment groups was observed, although no significant difference between treatment groups was seen. Combination therapy following monotherapy for 12 weeks showed significantly greater reduction in UACR (mean reduction of 50%) than candesartan cilexetil 16 mg monotherapy (mean reduction in UACR 24%) and numerically greater reduction than lisinopril 20 mg monotherapy (mean reduction in UACR 39%). All treatment regimens reduced both systolic and diastolic blood pressure significantly. The blood pressure reductions were significantly greater with combination therapy than with monotherapy, whether lisinopril was added to candesartan, or candesartan was added to lisinopril. (See Table 2.)
 The antihypertensive effects of candesartan cilexetil and losartan potassium at their highest recommended doses administered once daily were compared in two randomised, double blind trials. In a total of 1,268 patients with mild to moderate hypertension who were not receiving other antihypertensive therapy, candesartan cilexetil 32 mg lowered systolic and diastolic blood pressure by 2 to 3 mmHg on average more than losartan potassium 100 mg, when measured at the time of either peak or trough effect.
The antihypertensive effects of candesartan cilexetil and losartan potassium at their highest recommended doses administered once daily were compared in two randomised, double blind trials. In a total of 1,268 patients with mild to moderate hypertension who were not receiving other antihypertensive therapy, candesartan cilexetil 32 mg lowered systolic and diastolic blood pressure by 2 to 3 mmHg on average more than losartan potassium 100 mg, when measured at the time of either peak or trough effect.
Heart failure. In patients with chronic heart failure (CHF) and depressed left ventricular systolic function (left ventricular ejection fraction, LVEF less than or equal to 40%), candesartan cilexetil decreases systemic vascular resistance and pulmonary capillary wedge pressure, increases plasma renin activity and angiotensin II concentration, and decreases aldosterone levels.
Treatment with candesartan cilexetil reduces mortality and hospitalisation due to CHF and improves symptoms as shown in the Candesartan in Heart failure - Assessment of Reduction in Mortality and morbidity (CHARM) program comprising three studies (CHARM-Alternative, CHARM-Added and CHARM-Preserved). In all three studies, patients on optimal baseline therapy were randomised to placebo or candesartan cilexetil (titrated from 4 or 8 mg once daily to 32 mg once daily or the highest tolerated dose, mean dose 24 mg) and followed for a median of 37.7 months.
CHARM-Alternative.
CHARM-Alternative was a multinational, randomised, double blind placebo controlled study in CHF patients (New York Heart Association (NHYA) class II to IV, n = 2,028) with a LVEF less than or equal to 40% not treated with an ACE inhibitor because of intolerance. See Table 3.

CHARM-Added.
CHARM-Added was a multinational, randomised, double blind placebo controlled study in CHF patients (NYHA class II to IV, n = 2,548) with a LVEF less than or equal to 40% treated with ACE inhibitors. See Table 4.

CHARM-Preserved.
CHARM-Preserved was a multinational, randomised, double blind placebo controlled study in CHF patients (n = 3,023, NYHA class II to IV) with a LVEF > 40%, approximately 20% of whom received an ACE inhibitor. In the CHARM-Preserved study there was no effect of candesartan upon mortality. See Table 5.
 All-cause mortality was also assessed in pooled populations, CHARM-Alternative and CHARM-Added (HR 0.88, 95% CI: 0.79 to 0.98, p = 0.018) and all three studies (HR 0.91, 95% CI: 0.83 to 1.00, p = 0.055). This corresponds to a relative risk reduction of 12 and 9% respectively and an absolute risk reduction of 2.9 and 1.6% respectively.
All-cause mortality was also assessed in pooled populations, CHARM-Alternative and CHARM-Added (HR 0.88, 95% CI: 0.79 to 0.98, p = 0.018) and all three studies (HR 0.91, 95% CI: 0.83 to 1.00, p = 0.055). This corresponds to a relative risk reduction of 12 and 9% respectively and an absolute risk reduction of 2.9 and 1.6% respectively.
Treatment with candesartan cilexetil resulted in improved NYHA functional class in CHARM-Alternative and CHARM-Added (p = 0.008 and 0.020 respectively).
The beneficial effects of candesartan cilexetil on cardiovascular mortality and CHF hospitalisation were consistent irrespective of age, gender and concomitant medication. Candesartan cilexetil was effective also in patients taking both beta-blockers and ACE inhibitors at the same time, and the benefit was obtained whether or not patients were taking ACE inhibitors at the target dose recommended by treatment guidelines.
5.2 Pharmacokinetic Properties
Absorption and distribution.
Following oral administration, candesartan cilexetil is converted to the active drug candesartan. The absolute bioavailability of candesartan is approximately 40% after an oral solution of candesartan cilexetil. The relative bioavailability of the tablet formulation compared with the same oral solution is approximately 34%, with little variability. The absolute bioavailability of candesartan following administration of the tablet is approximately 14%. The mean peak serum concentration (Cmax) is reached three to four hours after taking a tablet. The point estimate of Cmax is 103.83% with associated confidence interval of [96.65%, 111.55%]. The candesartan serum concentrations increase linearly with increasing doses in the therapeutic dose range. The area under the serum concentration versus time curve (AUC) of candesartan is not significantly affected by food. The peak concentration (Cmax) is increased by 26% and the rate of absorption is increased when taken with food. These changes are unlikely to result in clinically significant effects.
In case of AUC0-t, the point estimate is 95.45% with associated confidence interval of [91.14%, 99.96%] and AUC0-∞ has point estimate of 94.96% and corresponding associated confidence interval of [90.73%, 99.37%].
Candesartan is highly bound to plasma protein (more than 99%). The apparent volume of distribution (Vss) of candesartan is 0.1 L/kg.
Metabolism and elimination.
Candesartan is mainly eliminated unchanged via urine and bile and is eliminated by hepatic metabolism only to a minor extent. The terminal half-life of candesartan is approximately nine hours. There is no accumulation following multiple doses.
Total plasma clearance of candesartan is about 0.37 mL/minute/kg, with a renal clearance of about 0.19 mL/minute/kg. The renal elimination of candesartan is both by glomerular filtration and active tubular secretion. Following an oral dose of 14C-labelled candesartan cilexetil about 30 and 70% of the total radioactivity is recovered in the urine and faeces, respectively.
Pharmacokinetics in special populations.
In the elderly (over 65 years), both Cmax and AUC of candesartan are increased in comparison to young subjects. An initial dose of 8 mg is recommended (see Section 4.2 Dose and Method of Administration).
In patients with mild to moderate renal impairment Cmax and AUC of candesartan increased during repeated dosing by approximately 50 and 70%, respectively, but t1/2 was not altered, compared to patients with normal renal function. The corresponding changes in patients with severe renal impairment were approximately 50 and 110%, respectively. The terminal t1/2 of candesartan was approximately doubled in patients with severe renal impairment. AUC of candesartan in patients undergoing haemodialysis was similar to that in patients with severe renal impairment.
In patients with mild to moderate hepatic impairment, there was a 23% increase in the AUC of candesartan. No initial dosage adjustment is necessary in these patients.
Following oral administration of BTC Candesartan 16 mg to healthy subjects under fasting conditions, a mean peak plasma concentration (Cmax) of candesartan of approximately 118.18 nanogram/mL was achieved within approximately 4.02 hours (Tmax).
5.3 Preclinical Safety Data
Genotoxicity.
Candesartan showed no evidence of genotoxic potential in a series of assay for gene mutations (Salmonella typhimurium, Escherichia coli, mouse L5178Y cells and CHO cells), chromosomal aberrations (mouse nucleus assay) and unscheduled DNA synthesis. The active metabolite, candesartan, caused an increase in chromosomal aberrations in vitro (CHL cells) but not in vivo (mouse micronucleus assay).
Carcinogenicity.
There was no evidence of carcinogenicity when candesartan cilexetil was orally administered to mice and rats for up to 104 weeks at doses up to 100 and 1000 mg/kg/day, respectively. Rats received the medicine by gavage whereas mice received the medicine by dietary administration. These (maximally tolerated) doses of candesartan cilexetil provided systematic exposures to candesartan (AUCs) that were, in mice, approximately 7 times and, in rats, more than 70 times the exposure in humans at the maximum recommended daily human dose (32 mg).6 Pharmaceutical Particulars
6.1 List of Excipients
Lactose monohydrate, iron oxide red (8 mg, 16 mg and 32 mg tablets only), titanium dioxide (8 mg, 16 mg and 32 mg tablets only), maize starch, povidone, carrageenan, croscarmellose sodium, magnesium stearate.
6.2 Incompatibilities
Incompatibilities were either not assessed or not identified as part of the registration of this medicine.
For information on interactions with other medicines and other forms of interactions, see Section 4.5.
6.3 Shelf Life
In Australia, information on the shelf life can be found on the public summary of the Australian Register of Therapeutic Goods (ARTG). The expiry date can be found on the packaging.
6.4 Special Precautions for Storage
Store below 30°C. Store in the original packaging.
6.5 Nature and Contents of Container
BTC Candesartan is available in Al/Al blister packs of 30 tablets.
6.6 Special Precautions for Disposal
In Australia, any unused medicine or waste material should be disposed of in accordance with local requirements.
6.7 Physicochemical Properties
Chemical structure.
The chemical name of Candesartan cilexetil is (±)-1-(cyclohexyloxycarbonyl-oxy) ethyl 2-ethoxy- 1-[[2'-(1H-tetrazol-5-yl)-biphenyl-4-yl]methyl]-1H-benzimadozole-7-carboxylate. Its empirical formula is C33H34N6O6 (MW: 610.7) and its chemical structure is:

CAS number.
145040-37-5.7 Medicine Schedule (Poisons Standard)
S4 - Prescription Only Medicine.
Summary Table of Changes





 The antihypertensive effects of candesartan cilexetil and losartan potassium at their highest recommended doses administered once daily were compared in two randomised, double blind trials. In a total of 1,268 patients with mild to moderate hypertension who were not receiving other antihypertensive therapy, candesartan cilexetil 32 mg lowered systolic and diastolic blood pressure by 2 to 3 mmHg on average more than losartan potassium 100 mg, when measured at the time of either peak or trough effect.
The antihypertensive effects of candesartan cilexetil and losartan potassium at their highest recommended doses administered once daily were compared in two randomised, double blind trials. In a total of 1,268 patients with mild to moderate hypertension who were not receiving other antihypertensive therapy, candesartan cilexetil 32 mg lowered systolic and diastolic blood pressure by 2 to 3 mmHg on average more than losartan potassium 100 mg, when measured at the time of either peak or trough effect.

 All-cause mortality was also assessed in pooled populations, CHARM-Alternative and CHARM-Added (HR 0.88, 95% CI: 0.79 to 0.98, p = 0.018) and all three studies (HR 0.91, 95% CI: 0.83 to 1.00, p = 0.055). This corresponds to a relative risk reduction of 12 and 9% respectively and an absolute risk reduction of 2.9 and 1.6% respectively.
All-cause mortality was also assessed in pooled populations, CHARM-Alternative and CHARM-Added (HR 0.88, 95% CI: 0.79 to 0.98, p = 0.018) and all three studies (HR 0.91, 95% CI: 0.83 to 1.00, p = 0.055). This corresponds to a relative risk reduction of 12 and 9% respectively and an absolute risk reduction of 2.9 and 1.6% respectively.
